 |
 |
| Korean J Intern Med > Volume 38(2); 2023 > Article |
|
This article has been corrected. See Korean J Intern Med. 2023 Jun 09; 38(4): 578.
Abstract
Background/Aims
Daratumumab has shown an encouraging antitumor effect in patients with multiple myeloma (MM), and was known to alter the immune properties by off-targeting immunosuppressive cells. Here, we aimed to evaluate the change in absolute lymphocyte count (ALC) as a surrogate marker for predicting survival outcomes of patients treated with daratumumab.
Methods
Between 2018 and 2021, the medical records of patients with relapsed/refractory MM (RRMM) treated with daratumumab monotherapy at 10 centers in South Korea were reviewed. We collected the ALC data at pre-infusion (D0), day 2 after the first infusion (D2), and prior to the third cycle of daratumumab therapy (D56).
Results
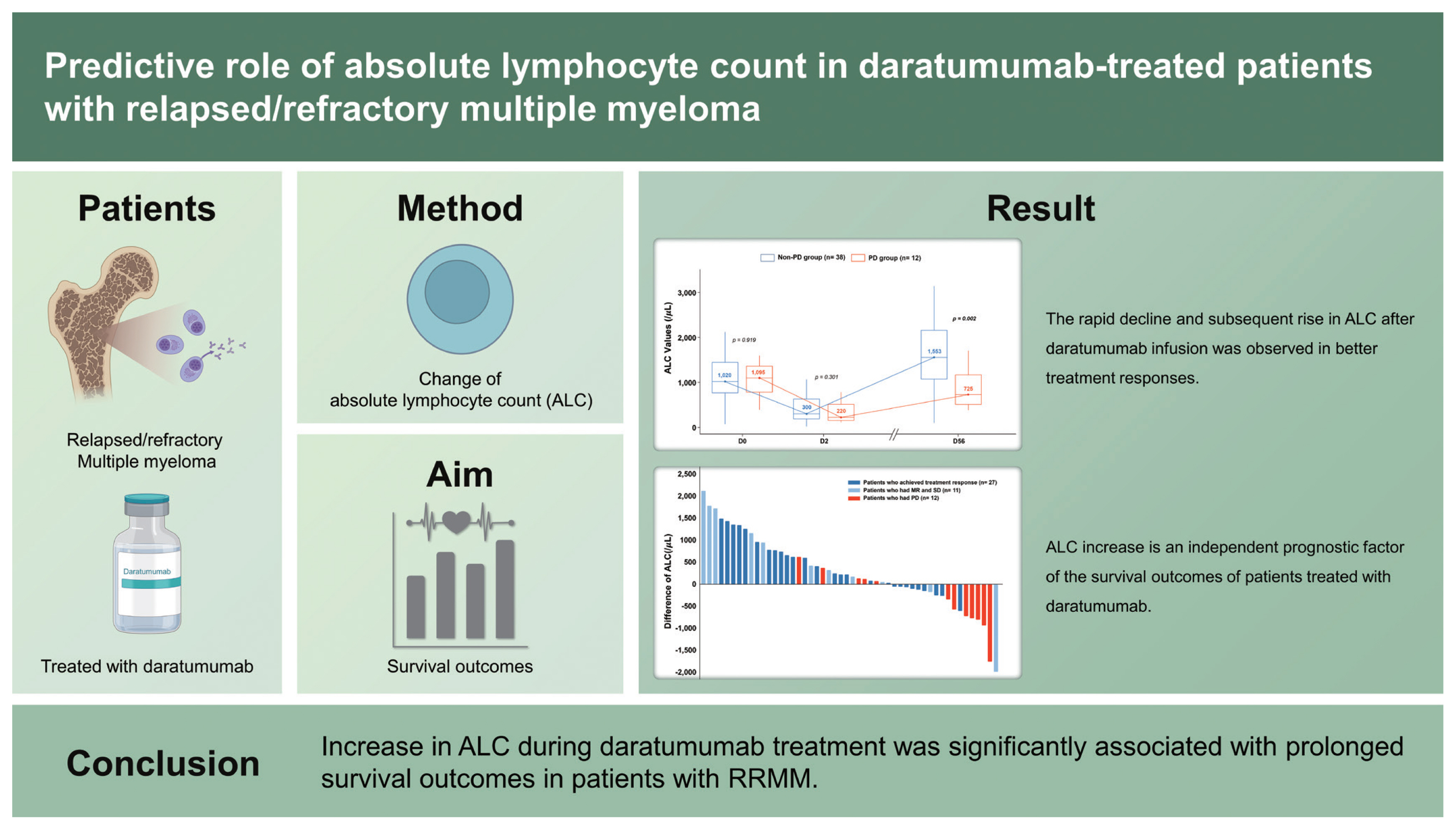
Fifty patients who were administered at least two cycles of daratumumab were included. Overall response rate was 54.0% after two cycles of daratumumab treatment. On D2, almost all patients experienced a marked reduction in ALC. However, an increase in ALC on D56 (ALCD56) was observed in patients with non-progressive disease, whereas failure of ALC recovery was noted in those with progressive disease. Patients with ALCD56 > 700/╬╝L (n = 39, 78.0%) had prolonged progression-free survival (PFS) and overall survival (OS) than those with ALCD56 Ōēż 700/╬╝L (median PFS: 5.8 months vs. 2.6 months, p = 0.025; median OS: 24.1 months vs. 6.1 months, p = 0.004). In addition, ALCD56 >700/╬╝L was a significant favorable prognostic factor for PFS (hazard ratio [HR], 0.22; p = 0.003) and OS (HR, 0.23; p = 0.012).
The introduction of novel agents, proteasome inhibitors (PI) and immunomodulatory drugs (IMiD), and high-dose chemotherapy followed by autologous stem cell transplantation (ASCT) have contributed to a substantial improvement in the survival outcomes of patients with multiple myeloma (MM) [1]. However, the majority of patients become relapsed and/or refractory (RR) despite novel agent-based treatment regardless of receiving ASCT, and the natural course of MM is considered incurable [2]. In particular, the survival outcomes of patient refractory to PI and/or IMiD are dismal, with a median survival of 8 to 9 months and limited treatment options available for these patients [3]. Therefore, it is imperative to develop a novel therapy that has antimyeloma mechanisms different from those of PIs and IMiDs.
Daratumumab selectively binds to CD38, which is ubiquitously and highly expressed in malignant plasma cells, and mediates cell death through various mechanisms, including antibody-dependent cell-mediated cytotoxicity, complement-dependent cytotoxicity, and antibody-dependent cellular phagocytosis [3]. In MM patients heavily pretreated with PI and/or IMiD, daratumumab treatment demonstrated promising results: an overall response rate (ORR) of 29% to 36% and a durable response duration of 6.9 to 7.6 months with a favorable safety profile [4ŌĆō6]. Addition of daratumumab to backbone therapy comprising PI and/or IMiD has shown remarkable efficacy in patients with newly diagnosed MM [7,8]; thus, its earlier use as a front-line treatment appears promising and is gradually increasing. However, despite the encouraging therapeutic efficacies of daratumumab, some patients failed to respond to the treatment. Furthermore, heterogeneity was observed in the duration and quality of treatment response in patients who responded to daratumumab.
Lymphocyte count has been associated with clinical outcomes in solid cancers [9] and several hematologic malignancies [10ŌĆō12]. As immunotherapy has been introduced in cancer management, the role of lymphocytes has been the hotbed of research as these cells reflect the host immune function, which determines the overall success of immune-based treatment [9]. Daratumumab was revealed to exert an immunomodulatory action by inhibiting CD38-expressing immunosuppressive cells, which might result in prolonged survival and delayed recurrence [13,14]. Given the immune-mediated action of daratumumab, it could be hypothesized that the lymphocyte count may be associated with the treatment outcomes of patients with MM treated with daratumumab. Therefore, in this study, we aimed to evaluate the changes in absolute lymphocyte count (ALC) in patients treated with daratumumab and to identify the effect of ALC on the treatment response and survival outcomes of these patients.
We retrospectively reviewed the medical records of patients diagnosed with MM according to the International Myeloma Working Group (IMWG) guidelines [15] at 10 medical centers in South Korea between December 2004 and June 2021. The inclusion criteria were as follows: (1) patients treated with daratumumab after more than three lines of therapy administered previously, including PI, IMiD, and/or ASCT, and (2) patients who received at least two cycles of daratumumab (total eight infusions) and underwent complete blood count (CBC) evaluation before and after daratumumab infusion. The exclusion criteria were as follows: (1) patients who received concurrent administration of other antimyeloma therapy with daratumumab and (2) patients with missing CBC values or whose treatment response was not assessed. This study was approved by the Institutional Review Board of Kyungpook National University Hospital (IRB no. 2021-05-13) and by each participating center in accordance with the Declaration of Helsinki. Written informed consent by the patients was waived due to a retrospective nature of our study.
Daratumumab was intravenously infused at a dose of 16 mg/kg weekly (days 1, 8, 15, and 22) for the first two cycles, every 2 weeks (days 1 and 15) for the subsequent four cycles, and every 4 weeks thereafter. According to the IMWG consensus criteria, treatment responses were classified as follows: complete response (CR), very good partial response (VGPR), partial response (PR), minimal response, stable disease (SD), and progressive disease (PD) [16]. The ORRs were CR, VGPR, and PR, respectively. Achieving SD or a better response was defined as non-progressive disease (non-PD). Response was evaluated after completing two cycles (eight infusions) of daratumumab.
The CBC assessment included evaluation of hemoglobin, white blood cell count, absolute neutrophil count (ANC), ALC, and platelet (PLT) count. To monitor the serial changes in CBC during daratumumab therapy, we collected the CBC results performed on the following days: D0, the day or the previous day of the first daratumumab infusion; D2, from the second to the fourth day after the first infusion; and D56, the day prior to starting the third cycle of daratumumab therapy (Supplementary Fig. 1). Unless the patients proceeded with the third cycle of daratumumab for any reason, the CBC for D56 was replaced with the results obtained within 7 days after completing the second cycle (eighth infusion) of daratumumab.
Data related to patient characteristics are presented as proportions and medians. Continuous variables were compared using a two-sample t test or analysis of variance, and categorical data were compared using the chi-square test. Progression-free survival (PFS) was defined as the time from diagnosis to disease progression. Overall survival (OS) was defined as the time from diagnosis to the time of the last follow-up or all-cause death. PFS and OS curves were plotted using the Kaplan-Meier method, and group comparisons were performed using a log-rank test. Prognostic factors affecting OS and PFS were evaluated using a Cox regression model. Factors with p < 0.1 in the univariate analysis were included in the multivariate analysis, and p < 0.05 were considered significant. A receiver operating characteristic (ROC) curve analysis was conducted to determine the optimal cut-off value of variables that predict treatment outcomes. R statistical software 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria; available at http://www.r-project.org) and SPSS version 20.0 (IBM Co., Armonk, NY, USA) were used for all statistical analyses.
Fifty patients with relapsed/refractory MM (RRMM) who received daratumumab monotherapy were included in the final analysis (Supplementary Fig. 2). The median age at the initiation of daratumumab therapy was 64.0 years (range, 40 to 86), and 24 patients (48.0%) were aged 65 years or older. Twenty-seven patients (54.0%) were male. The Eastern Cooperative Oncology Group (ECOG) performance status score was 0 or 1 in 40 patients (80.0%) and 2 or 3 in eight patients (16.0%). Immunoglobulin G (IgG) was detected in a majority of the patients (n = 36, 72.0%). IgA, light chain type, and unknown were found in five (10.0%), eight (16.0%), and one patient (2.0%), respectively. Of the total patients, 34 (68.0%) were classified as Revised International Staging System (R-ISS) I or II, and 10 (20.0%) were classified as the R-ISS III. Patients received a median of four (range, 3 to 7) previous lines of treatment before the initiation of daratumumab therapy. All patients were refractory to PI and/or IMiD and/or relapsed after receiving these regimens, 60% patients (n = 30) underwent ASCT. The median number of daratumumab treatment cycles was 4.5 (range, 2 to 25). Sixteen patients (32.0%) were still receiving daratumumab at the time of data analysis (Supplementary Table 1). In terms of the treatment response after completing the second cycle of daratumumab, the observed responses were as follows: PR or better in 27 patients (54 %), CR in two (4.0%), VGPR in six (12.0%), and PR in 19 patients (38.0%). Meanwhile, 12 patients (24.0%) experienced progression after two cycles of daratumumab. The detailed patient characteristics and treatment outcomes are summarized in Table 1.
The median ALC value was 1,070/╬╝L (range, 70 to 3,430) on D0 but sharply dropped to 295/╬╝L (range, 20 to 3,100) on D2. After two cycles of daratumumab therapy, the ALC value recovered to 1,388/╬╝L (range, 100 to 3,140) (Table 1). Meanwhile, the hemoglobin value, ANC, and PLT counts followed a trend different from that of ALC during the course of treatment, although the PLT count slightly decreased on D2 (Supplementary Table 2).
An ROC plot was generated to determine the optimal ALC value based on the overall response of the patients (PR or better), and the cut-off value for ALC on D56 (ALCD56) was determined as 700/╬╝L (area under the curve = 0.668; p = 0.023) (Supplementary Fig. 3). No association was found between the ALC value on D0 or D2 and treatment response. Overall, 39 (78.0%) and 11 (22.0%) patients had an ALCD56 > 700/╬╝L (ALCD56 > 700) and ALCD56 Ōēż 700/╬╝L (ALCD56 Ōēż 700), respectively. The distribution of each characteristic was not significantly different according to the ALCD56 value (Table 1); however, patients with ALCD56 > 700 received more cycles of daratumumab therapy (Supplementary Fig. 4) and showed a higher baseline ALC value than those with ALCD56 Ōēż 700 (Table 1). Interestingly, the ALC value increased from 1,120 to 1,600/╬╝L in patients with ALCD56 > 700 during daratumumab treatment but decreased in those with ALCD56 Ōēż 700 (Table 1). Among the other components of CBC, the PLT count showed a trend similar to that of the ALC value during daratumumab treatment (Supplementary Table 2).
We evaluated the changes in the ALC values of patients according to treatment response: non-PD or PD. In 38 patients (76.0%) who had SD or better (i.e., non-PD) ALC values changed dynamically: 1,020, 300, and 1,553/╬╝L on D0, D2, and D56, respectively (Fig. 1A). In contrast, for patients with PD (n = 12, 24%), the median ALC values did not completely recover after two cycles of daratumumab treatment: 1,095, 220, and 725/╬╝L on D0, D2, and D56, respectively (Fig. 1A). Between the patients with non-PD and PD, no difference was observed in the baseline ALC values (p = 0.919). However, a noticeable difference in ALC values was identified on D56 (p = 0.002) (Fig. 1A). A waterfall plot showed a difference in ALC values from D56 to D0 in all patients; most patients with non-PD (n = 27, 71.1% [27/38]) showed an increase in ALC values after receiving daratumumab therapy, whereas those with PD showed a decrease in ALC values (n = 7, 58% [7/12]) as shown in Fig. 1B. The changes in ALC values of each patient on D0, D2, and D56 are shown in Supplementary Fig. 5. Meanwhile, no significant changes in ANC and PLT values during daratumumab therapy were observed (Supplementary Fig. 6).
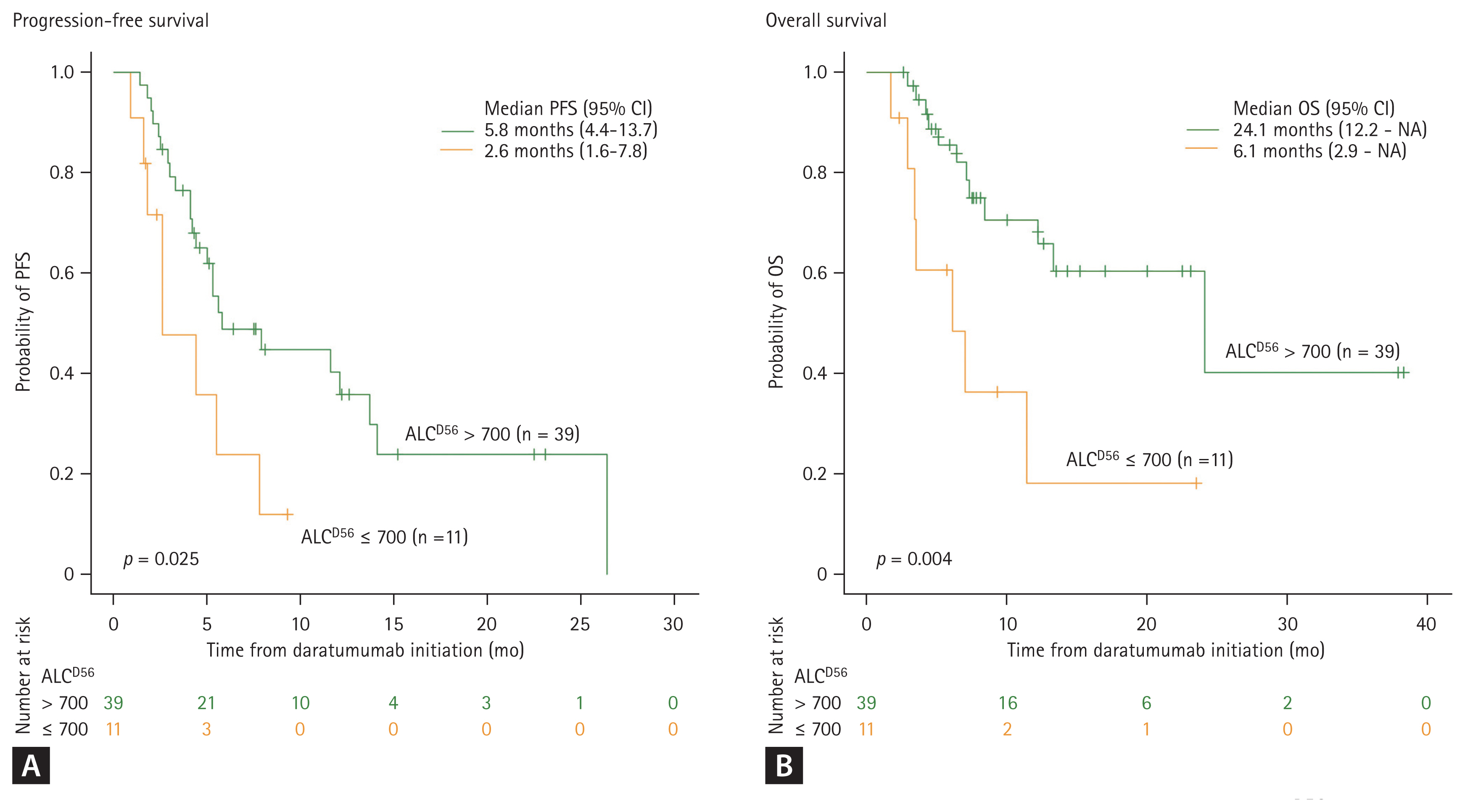
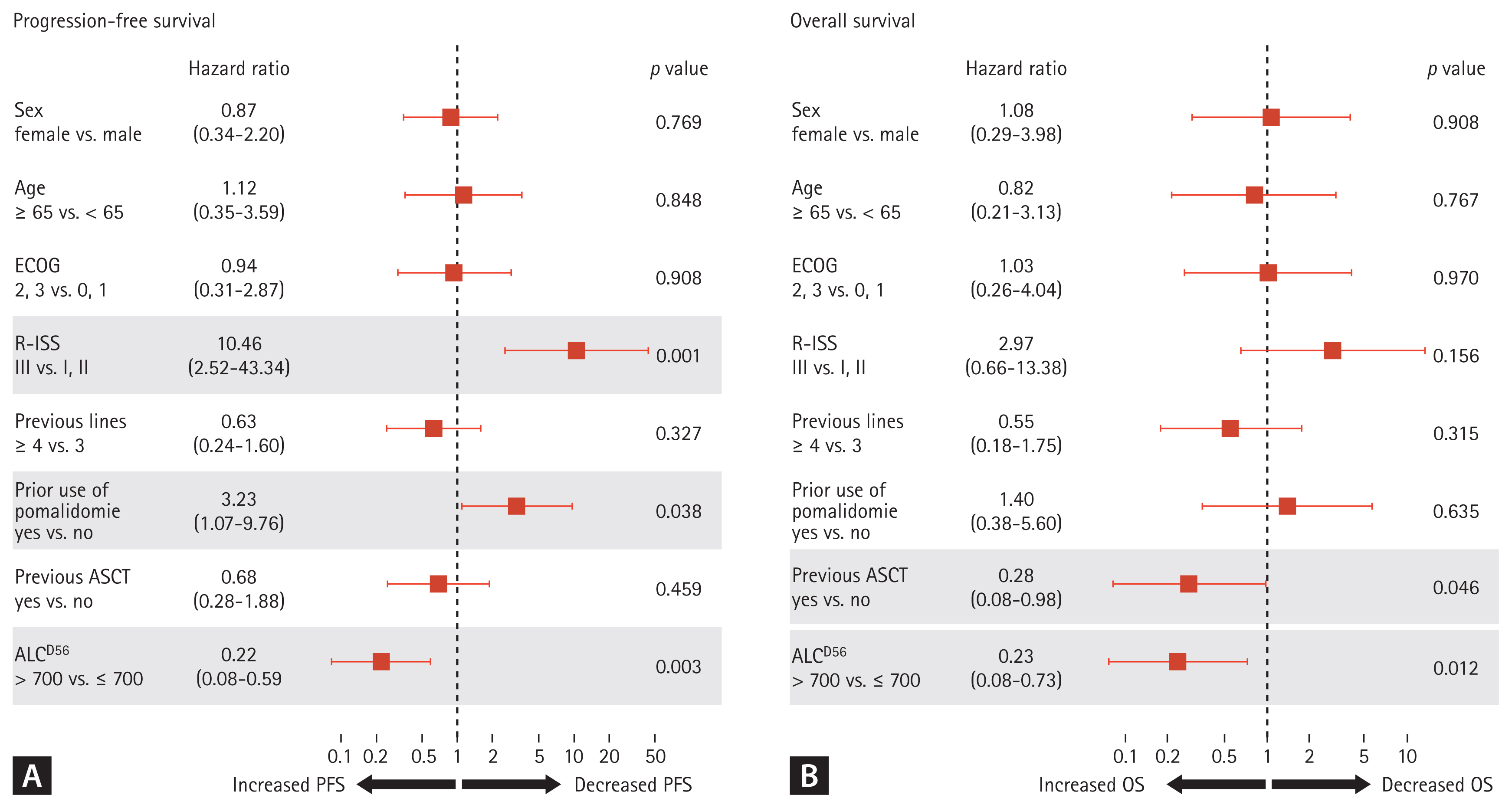
With a median follow-up of 7.2 months (range, 38.3 to 1.7), the survival outcomes were distinctively different based on the ALCD56 value. The median PFS was 5.8 months (range, 4.4 to 13.7) and 2.6 months (range, 1.6 to 7.8) for patients with ALCD56 > 700 and ALCD56 Ōēż 700, respectively (p = 0.025). Meanwhile, the median OS was 24.1 months (range, 12.2 to not applicable [NA]) and 6.1 months (range, 2.9 to NA) for patients with ALCD56 >700 and Ōēż 700, respectively (p = 0.004) (Fig. 2). Among patients with ALCD56 Ōēż 700, two were still receiving daratumumab at the time of data analysis; however, half of them died without subsequent treatment (Supplementary Table 1). In the univariate analysis, the following factors contributed to survival outcomes: sex, age, ECOG, R-ISS, number of previous lines of treatment, prior use of pomalidomide, previous ASCT, and ALCD56 value. In the multivariate analysis, ALCD56 > 700 was significantly associated with prolonged PFS (hazard ratio [HR], 0.22; 95% confidence interval [CI], 0.08 to 0.59; p = 0.003) and OS (HR, 0.23; 95% CI, 0.08 to 0.73; p = 0.012), as shown in Fig. 3. The other factors that contributed to the reduction in PFS were R-ISS stage III (HR, 10.46; 95% CI, 2.52 to 43.34; p = 0.001) and prior use of pomalidomide (HR, 3.23; 95% CI, 1.07 to 9.76; p = 0.038) (Fig. 3A). Patients who underwent ASCT prior to daratumumab treatment showed a lower risk of death (HR, 0.28; 95% CI, 0.08 to 0.98; p = 0.046) (Fig. 3B).
This study evaluated the dynamic changes in ALC values and the prognostic impact of ALC increase on the survival outcomes of patients treated with daratumumab. Almost all the patients experienced a rapid decline in ALC immediately after the first infusion of daratumumab, regardless of treatment response. However, the subsequent changes in the ALC values in response to further infusions of daratumumab were significantly different according to the treatment response; failure of ALC recovery was noted in patients with PD after daratumumab treatment, whereas the ALC value increased in those without PD. This immediate decline in ALC may be associated with a marked reduction in CD38 expression in plasma cells and non-plasma immune cells [17] or could be transiently influenced by the pre-infusion of steroids to manage infusion-related reactions to daratumumab [18]. However, the difference in ALC change according to treatment response may be involved in the immunomodulatory role of daratumumab. In a study evaluating regulatory T cells (Tregs) in patients treated with daratumumab, the absolute number of CD38-expressing Tregs before treatment was higher in patients who responded to daratumumab compared to those who did not responded to the therapy [19]. Indeed, an increase in the absolute number of T cells such as CD3+, CD4+, and CD8+ from baseline, which may be a consequence of Treg suppression by daratumumab treatment, was observed in daratumumab responders [13]. Taken together, the difference in ALC values between patients with and without PD could be attributed to the collective immune response modulated by daratumumab.
The host immune status determines treatment outcomes and has become more significant in the era of immune- based therapy. Therefore, an easily measurable and reliable immune marker is required to predict the clinical outcomes of patients undergoing immunotherapy. In patients with cancer, ALC is an established immune marker for predicting survival outcomes [9]. Several studies have reported its prognostic role in patients with MM. A retrospective analysis of 537 patients with MM demonstrated that an ALC > 1.4 ├Ś 109/L at diagnosis was associated with better OS [20]. After induction therapy, patients with an ALC > 0.8 ├Ś 109/L also had longer OS [21]. Additionally, early recovery of ALC after ASCT is an independent prognostic factor for OS [22]. In this study, the role of ALC as a surrogate marker for survival in patients treated with daratumumab was confirmed. Our study followed the serial changes in ALC values and showed an increasing trend of ALC in patients without PD. Furthermore, ALCD56 > 700/╬╝L after the second cycle of daratumumab was a significant prognostic factor for patient survival outcomes.
In this study, patients who previously received pomalidomide before the initiation of daratumumab therapy had a shorter PFS than those who did not. However, the underlying mechanisms remain unknown. Because therapeutic efficacy of daratumumab has been observed in IMiD-refractory or -relapsed patients in clinical trials [4,5]. Considering that the addition of IMiD to daratumumab-refractory MM patients can act synergistically to overcome drug resistance [23], the expected alteration in immune properties by treatment with a single IMiD might be insufficient to prime an immunological environment to fight against malignant plasma cells. In another explanation, the sequence of therapy may be important in heavily treated patients with MM. A previous single-center retrospective observational study of patients with RRMM showed that patients who were retreated with IMiDs and PIs after daratumumab monotherapy had impressive ORRs of 52% and 67%, respectively [24]. Additionally, an increase in ALC, which reflects the immune status of the patients, would warrant the use of IMiDs or cellular therapies after daratumumab treatment to improve long-term outcomes.
Despite presenting some credible findings, this study had several limitations. First, we did not perform flow cytometry analysis to identify the type and proportion of subsets consisting of ALC, although we postulated that an increase in ALC during daratumumab treatment might be associated with a favorable immune outcome, such as T cell expansion. Second, changes in ALC values were not identified in patients who received combination therapy. As the use of daratumumab in combination with IMiD and/or PI is increasing, the clinical relevance of ALC in survival outcomes should also be verified in patients treated with three or four regimens. Lastly, medical conditions affecting the CBC value may concurrently exist during daratumumab treatment. Owing to the retrospective nature of this study, we could not control such conditions that may alter the ALC values. However, patients with medical conditions, such as uncontrolled infections that could change the immune profiles, were excluded.
Nevertheless, our findings suggest that ALC is a potential surrogate marker for predicting survival outcomes in patients undergoing daratumumab-based therapy. ALC increase was an independent prognostic factor for survival outcomes in patients treated with daratumumab. Moreover, persistent decrease in ALC values after daratumumab therapy significantly correlated with unfavorable treatment outcomes.
1. The rapid decline and subsequent rise in absolute lymphocyte count (ALC) after daratumumab infusion was observed in multiple myeloma patients with better treatment responses.
2. ALC increase is an independent prognostic factor of the survival outcomes of patients treated with daratumumab.
3. ALC could be a surrogate marker for predicting the survival outcomes of patients treated with daratumumab.
Figure┬Ā1
Changes in absolute lymphocyte count (ALC) values during daratumumab therapy. (A) Box plot depicting changes in ALC values according to treatment response. Each pair of boxes shows the ALCD0, ALCD2, and ALCD56 values of patients treated with daratumumab. (B) Waterfall plot of differences in ALC values in all patients. The values on the y-axis indicate the differences in the ALC values (from ALCD56 to ALCD0). PD, progressive disease; MR, minimal response; SD, stable disease.

Figure┬Ā2
Survival analysis of each absolute lymphocyte counts at the day prior to the third cycle of daratumumab (ALCD56) value after daratumumab treatment. (A) Progression-free survival (PFS), (B) overall survival (OS). NA, not applicable.
Figure┬Ā3
Multivariate Cox regression analyses. (A) Progression-free survival (PFS), (B) overall survival (OS). ECOG, Eastern Cooperative Oncology Group; R-ISS, Revised International Staging System; ASCT, autologous stem cell transplantation; ALC, absolute lymphocyte count.
Table┬Ā1
Baseline characteristics of patients and clinical outcomes after daratumumab
| Characteristic | Overall (n = 50) | ALC at day 56 after daratumumab | p value | |
|---|---|---|---|---|
| > 700/╬╝L (n = 39) | Ōēż 700/╬╝L (n = 11) | |||
| Age, yr | 64.0 (40ŌĆō86) | 63.8 (40ŌĆō86) | 67.0 (53ŌĆō82) | 0.623 |
| ŌĆāAge Ōēź 65 | 24 (48.0) | 18 (46.2) | 6 (54.5) | |
| Sex | 0.520 | |||
| ŌĆāMale | 27 (54.0) | 22 (56.4) | 5 (45.5) | |
| ŌĆāFemale | 23 (46.0) | 17 (44.6) | 6 (54.5) | |
| ECOG PS | 0.411 | |||
| ŌĆā0ŌĆō1 | 40 (80.0) | 32 (82.1) | 8 (72.7) | |
| ŌĆā2ŌĆō3 | 8 (16.0) | 5 (12.8) | 3 (27.3) | |
| ŌĆāUnknown | 2 (4.0) | 2 (5.1) | 0 (0.0) | |
| Subtype | 0.080 | |||
| ŌĆāIgG | 36 (72.0) | 28 (71.8) | 8 (72.7) | |
| ŌĆāIgA | 5 (10.0) | 2 (5.1) | 3 (27.3) | |
| ŌĆāLight chain type | 8 (16.0) | 8 (20.5) | 0 | |
| ŌĆāUnknown | 1 (2.0) | 1 (2.6) | 0 | |
| ISS | 0.056 | |||
| ŌĆāI, II | 25 (50.0) | 22 (56.4) | 3 (27.3) | |
| ŌĆāIII | 21 (42.0) | 13 (33.3) | 8 (72.7) | |
| ŌĆāUnknown | 4 (8.0) | 4 (10.3) | 0 | |
| R-ISS | 0.348 | |||
| ŌĆāI, II | 34 (68.0) | 26 (66.7) | 8 (72.7) | |
| ŌĆāIII | 10 (20.0) | 7 (17.9) | 3 (27.3) | |
| ŌĆāUnknown | 6 (12.0) | 6 (15.4) | 0 | |
| Previous lines of therapy | 4 (3ŌĆō7) | 4 (3ŌĆō7) | 4 (3ŌĆō5) | 1.000 |
| ŌĆā3 lines | 18 (36.0) | 14 (35.9) | 4 (36.4) | |
| ŌĆā4ŌĆō7 lines | 32 (64.0) | 25 (64.1) | 7 (63.6) | |
| Prior use of PI | 50 (100.0) | 39 (100) | 11 (100) | |
| ŌĆāBortezomib | 48 (96.0) | 38 (97.4) | 10 (90.9) | 0.395 |
| ŌĆāCarfilzomib | 26 (52.0) | 16 (41.0) | 10 (90.9) | 0.003 |
| Prior use of IMiD | 50 (100.0) | 39 (100) | 11 (100) | |
| ŌĆāThalidomide | 34 (86.0) | 27 (69.2) | 7 (63.6) | 0.728 |
| ŌĆāLenalidomide | 49 (98.0) | 38 (97.4) | 11 (100.0) | 1.000 |
| ŌĆāPomalidomide | 30 (60.0) | 25 (64.1) | 5 (45.5) | 0.331 |
| Previous ASCT | 30 (60.0) | 25 (64.1) | 5 (45.5) | 0.311 |
| Cycles of daratumumab | 4.5 (2ŌĆō25) | 5 (2ŌĆō25) | 3 (2ŌĆō11) | 0.004 |
| Responsea | 0.093 | |||
| ŌĆāCR | 2 (4.0) | 2 (5.1) | 0 | |
| ŌĆāVGPR | 6 (12.0) | 5 (12.8) | 1 (9.1) | |
| ŌĆāPR | 19 (38.0) | 18 (20.5) | 1 (9.1) | |
| ŌĆāMR | 4 (8.0) | 3 (7.7) | 1 (9.1) | |
| ŌĆāSD | 7 (14.0) | 5 (12.8) | 2 (18.2) | |
| ŌĆāPD | 12 (24.0) | 6 (15.4) | 6 (54.5) | |
| Overall response rate | 27 (54.0) | 25 (64.1) | 2 (18.2) | 0.018 |
| ALC at D0, /╬╝L | 1,070 (70ŌĆō3,430) | 1,120 (330ŌĆō3,430) | 800 (70ŌĆō1,590) | 0.024 |
| ALC at D2, /╬╝L | 295 (20ŌĆō3,100) | 300 (40ŌĆō3,100) | 240 (20ŌĆō780) | 0.198 |
| ALC at D56, /╬╝L | 1,388 (100ŌĆō3,140) | 1,600 (720ŌĆō3,140) | 510 (100ŌĆō700) | < 0.001 |
ALC, absolute lymphocyte count; ECOG PS, Eastern Cooperative Oncology Group performance status; Ig, immunoglobulin; ISS, International Staging System; R-ISS, Revised International Staging System; PI, proteasome inhibitor; IMiD, immunomodulatory drug; ASCT, autologous stem cell transplantation; CR, complete response; VGPR, very good partial response; PR, partial response; MR, minimal response; SD, stable disease; PD, progressive disease.
REFERENCES
1. Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood 2008;111:2516ŌĆō2520.




2. Kumar SK, Lee JH, Lahuerta JJ, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia 2012;26:149ŌĆō157.

3. Blair HA. Daratumumab: a review in relapsed and/or refractory multiple myeloma. Drugs 2017;77:2013ŌĆō2024.



4. Lokhorst HM, Plesner T, Laubach JP, et al. Targeting CD38 with daratumumab monotherapy in multiple myeloma. N Engl J Med 2015;373:1207ŌĆō1219.


5. Lonial S, Weiss BM, Usmani SZ, et al. Daratumumab monotherapy in patients with treatment-refractory multiple myeloma (SIRIUS): an open-label, randomised, phase 2 trial. Lancet 2016;387:1551ŌĆō1560.


6. Usmani SZ, Weiss BM, Plesner T, et al. Clinical efficacy of daratumumab monotherapy in patients with heavily pretreated relapsed or refractory multiple myeloma. Blood 2016;128:37ŌĆō44.




7. Facon T, Kumar S, Plesner T, et al. Daratumumab plus lenalidomide and dexamethasone for untreated myeloma. N Engl J Med 2019;380:2104ŌĆō2115.


8. Voorhees PM, Kaufman JL, Laubach J, et al. Daratumumab, lenalidomide, bortezomib, and dexamethasone for transplant-eligible newly diagnosed multiple myeloma: the GRIFFIN trial. Blood 2020;136:936ŌĆō945.




9. Menetrier-Caux C, Ray-Coquard I, Blay JY, Caux C. Lymphopenia in cancer patients and its effects on response to immunotherapy: an opportunity for combination with cytokines? J Immunother Cancer 2019;7:85.


10. Kim DH, Kim JG, Sohn SK, et al. Clinical impact of early absolute lymphocyte count after allogeneic stem cell transplantation. Br J Haematol 2004;125:217ŌĆō224.



11. Siddiqui M, Ristow K, Markovic SN, et al. Absolute lymphocyte count predicts overall survival in follicular lymphomas. Br J Haematol 2006;134:596ŌĆō601.


12. De Angulo G, Yuen C, Palla SL, Anderson PM, Zweidler-McKay PA. Absolute lymphocyte count is a novel prognostic indicator in ALL and AML: implications for risk stratification and future studies. Cancer 2008;112:407ŌĆō415.


13. Krejcik J, Casneuf T, Nijhof IS, et al. Daratumumab depletes CD38+ immune regulatory cells, promotes T-cell expansion, and skews T-cell repertoire in multiple myeloma. Blood 2016;128:384ŌĆō394.




14. van de Donk NW, Usmani SZ. CD38 antibodies in multiple myeloma: mechanisms of action and modes of resistance. Front Immunol 2018;9:2134.



15. Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol 2014;15:e538ŌĆōe548.


16. Kumar S, Paiva B, Anderson KC, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol 2016;17:e328ŌĆōe346.


17. Krejcik J, Frerichs KA, Nijhof IS, et al. Monocytes and granulocytes reduce CD38 expression levels on myeloma cells in patients treated with daratumumab. Clin Cancer Res 2017;23:7498ŌĆō7511.




18. Fauci AS, Dale DC, Balow JE. Glucocorticosteroid therapy: mechanisms of action and clinical considerations. Ann Intern Med 1976;84:304ŌĆō315.


19. Kitadate A, Kobayashi H, Abe Y, et al. Pre-treatment CD38-positive regulatory T cells affect the durable response to daratumumab in relapsed/refractory multiple myeloma patients. Haematologica 2020;105:e37ŌĆōe40.



20. Ege H, Gertz MA, Markovic SN, et al. Prediction of survival using absolute lymphocyte count for newly diagnosed patients with multiple myeloma: a retrospective study. Br J Haematol 2008;141:792ŌĆō798.


21. Narwani V, Gabriel J, Boyd K, Chevassut T. Absolute lymphocyte count at day 29 of treatment is a powerful predictor of outcome in multiple myeloma. Clin Lymphoma Myeloma Leuk 2015;15:222ŌĆō226.


22. Jimenez-Zepeda VH, Reece DE, Trudel S, et al. Absolute lymphocyte count as predictor of overall survival for patients with multiple myeloma treated with single autologous stem cell transplant. Leuk Lymphoma 2015;56:2668ŌĆō2673.


- TOOLS
-
METRICS

-
- 1 Web of Science
- 0 Crossref
- 0 Scopus
- 545 View
- 152 Download
- Related articles



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement figure 1
Supplement figure 1 Print
Print


