 |
 |
| Korean J Intern Med > Volume 38(3); 2023 > Article |
|
Abstract
Background/Aims
Although the conversion from tacrolimus (TAC) to cytotoxic T-lymphocyte-associated antigen 4-immunoglobulin (CTLA4-Ig) is effective in reducing TAC-induced nephrotoxicity, it remains unclear whether CTLA4-Ig has a direct effect on TAC-induced renal injury. In this study, we evaluated the effects of CTLA4-Ig on TAC-induced renal injury in terms of oxidative stress.
Methods
in vitro study was performed to assess the effect of CTLA4-Ig on TAC-induced cell death, reactive oxygen species (ROS), apoptosis, and the protein kinase B (AKT)/forkhead transcription factor (FOXO) 3 pathway in human kidney 2 cells. In the in vivo study, the effect of CTLA4-Ig on TAC-induced renal injury was evaluated using renal function, histopathology, markers of oxidative stress (8-hydroxy-2ŌĆÖ-deoxyguanosine) and metabolites (4-hydroxy-2-hexenal, catalase, glutathione S-transferase, and glutathione reductase), and activation of the AKT/FOXO3 pathway with insulin-like growth factor 1 (IGF-1).
Results
CTLA4-Ig significantly decreased cell death, ROS, and apoptosis caused by TAC. TAC treatment increased apoptotic cell death and apoptosis-related proteins (increased Bcl-2-associated X protein and caspase-3 and decreased Bcl-2), but it was reversed by CTLA4-Ig treatment. The activation of p-AKT and p-FOXO3 by TAC decreased with CTLA4-Ig treatment. TAC-induced renal dysfunction and oxidative marker levels were significantly improved by CTLA4-Ig in vivo. Concomitant IGF-1 treatment abolished the effects of CTLA4-Ig.
Tacrolimus (TAC), a calcineurin inhibitor used as a first-line drug after renal transplantation, prominently reduces the incidence of acute rejection following renal transplantation [1,2]. However, long-term use of TAC can cause chronic transplant kidney injury, which is one of the reasons for chronic transplant kidney failure [3-5]. The pathogenesis of TAC-induced nephrotoxicity is multifactorial; however, TAC-induced oxidative stress is a common pathway for TAC-induced renal injury. TAC can directly cause renal bulbous artery stenosis and lead to ischemia and hypoxia injury, which results in the production of reactive oxygen species (ROS) and reduction of antioxidant enzymes (such as superoxide dismutase and glutathione catalase), thus inducing oxidative stress injury and ultimately leading to renal tubular cell apoptosis [6,7].
Cytotoxic T-lymphocyte-associated antigen 4-immunoglobulin (CTLA4-Ig) is a soluble CTLA4 protein formed by the fusion of the extracellular functional domain of CTLA4 with the crystallizable fragment of Ig, which binds with B7 with high affinity and can effectively block the costimulatory signal [8-10]. CTLA4-Ig is a novel immunosuppressant that can replace calcineurin inhibitors for anti-rejection therapy in patients with kidney transplantation [11], but its effect on TAC-induced renal injury is still unknown. We previously reported that conversion to CTLA4-Ig reduced TAC-induced pancreatic islet injury in diabetic rats and that CTLA4-Ig directly decreased TAC-induced pancreatic islet cell death and ROS production in vitro [12].
Forkhead transcription factor (FOXO) 3 is a conserved transcriptional factor, and the activation of FOXO3 is involved in inflammation, autophagy, oxidative stress, and cell death [13]. FOXO3 is modulated by the phosphatidylinositol 3ŌĆækinase (PI3K)/protein kinase B (AKT) pathway [14]. Furthermore, inhibition of the PI3K/AKT pathway can inhibit inflammation, and the inflammatory response of the obstructive kidney was reduced by regulating the AKT/FOXO3 pathway [15]. Hence, we speculated that the AKT/FOXO3 pathway may participate in the process of TAC-induced renal injury.
This study aimed to investigate the protective effect of CTLA4-Ig against TAC-induced renal injury and its related mechanism in established chronic TAC-induced nephrotoxicity in vivo and in vitro.
Human kidney 2 (HK-2) cells purchased from the Cell Bank of the Chinese Academy of Sciences were cultured in keratinocyte serum-free medium (K-SFM; 17005-042, Invitrogen, Carlsbad, CA, USA) with 10% fetal bovine serum (FBS; 10099-141, Gibco, Carlsbad, CA, USA), and gentamicin/amphotericin solution 500├Ś (R-015-10, Gibco). The cells were cultured in an incubator at 37┬░C, with 5% CO2 and 95% humidity for 24 hours. HK-2 cells were pretreated with CTLA4-Ig (5 ╬╝g/mL, Bristol-Myers-Squibb, New York, NY, USA), and then co-treated with TAC (1642802, 0, 20, 40, 80 ╬╝g/mL; Sigma, Milpitas, CA, USA) and insulin-like growth factor 1 (IGF-1; an agonist for PI3K/AKT, 10 nM; Genentech, Southern San Francisco, CA, USA) [16].
Seventy male Sprague-Dawley rats (body weight 200ŌĆō220 g) were fed a low-salt diet (0.05% sodium salt) for 1 week, and were divided into seven groups with 10 rats in each group, using the random number table method. The experiment lasted for 6 weeks. TAC was administered subcutaneously once daily. CTLA4-Ig and IGF-1 were administered once a week through the tail vein of rats. The doses of drugs and the route of administration were chosen based on previous studies [12,17]. The timeline of all interventions is listed in Supplementary Figure 1. The specific groups were as follows: 1) vehicle group (n=10): subcutaneously injected with olive oil (1.0 mg/kg/day) for 6 weeks; 2) TAC group: we first selected the dose of TAC (0, 0.25, 0.5, and 1.0 mg/kg/day subcutaneously) for 6 weeks in 10 rats in each group based on renal injury indexes. Then 1.0 mg/kg/day TAC was selected for subsequent experiments; 3) conversion from TAC to olive oil (TV) group (n=10): subcutaneously injected with TAC (1.0 mg/kg/day) for the first 3 weeks followed by subcutaneous injection of olive oil (1.0 mg/kg/day) for an additional 3 weeks; 4) conversion from TAC to 1 mg/kg of CTLA4-Ig group (n=10): subcutaneously injected with TAC (1.0 mg/kg/day) for the first 3 weeks followed by tail vein injection of CTLA4-Ig (1.0 mg/kg/week) for an additional 3 weeks; and 5) conversion from TAC to 1 mg/kg of CTLA4-Ig along with IGF-1 group (n=10): subcutaneously injected with TAC (1.0 mg/kg/day) for the first 3 weeks followed by tail vein injection of CTLA4-Ig (1.0 mg/kg/week) along with IGF-1 (2.0 mg/kg/day) for an additional 3 weeks [18,19].
Peripheral blood was collected before the rats were sacrificed. After the rats were sacrificed, the intact kidney tissue was fixed using periodate acid-lysine-paraformaldehyde solution; the sections were 4 ╬╝m thick after paraffin embedding for unified detection. This study was approved by the Animal Ethical and Welfare Committee of The First Affiliated Hospital of Dalian Medical University (No. MDKN-2021-057).
The viability of HK-2 cells was analyzed using CCK-8 (enhanced CCK-8 kit; Beyotime, Shanghai, China). The cells were digested with 0.25% trypsin (T4549; Sigma), centrifuged, and resuspended in 200 ╬╝L of cell culture medium. Cells (1├Ś103) were seeded in a 96-well plate and incubated overnight at 37┬░C with 5% CO2. Cells in each group were incubated with 10 ╬╝L of CCK-8 solution for 1.5 hours at 24, 48, or 72 hours, and the absorbance at 450 nm was then detected using a microplate reader (BioTek, Vinusky, VT, USA).
The HK-2 adherent cells in the good growth state in each group were digested with trypsin, and the supernatant was discarded. A dichloro-dihydro-fluorescein diacetate fluorescent probe (ROS probe; Solarbio, Beijing, China) was added and incubated at room temperature for 20 minutes. After centrifugation, the supernatant was discarded, and phosphate buffered saline (Biological Industries, Shanghai, China) containing 1% FBS was added for resuspension. For the evaluation of cell apoptosis, Annexin V and 7-aminoactinomycin (7-AAD) double-staining was performed using the Annexin V Apoptosis Detection Kit fluorescein isothiocyante and 7-AAD Viability Staining Solution (eBioscience, San Diego, CA, USA) according to the manufacturerŌĆÖs instructions. The cells were measured using a flow cytometer (BD bioscience, San Diego, CA, USA) and analyzed using FlowJo software (Cabit Information Technology Co., Ltd., Shanghai, China). Apoptotic cells were considered as Annexin V-positive groups.
Cell suspension was prepared and the cells were treated according to the preset drug concentration and time. Sodium dodecyl sulfate lysis buffer was added, and the cells were placed on ice for 20 minutes. Then, the protein concentration was measured using the bicinchoninic acid assay method, the sample was loaded and transferred to the membrane after electrophoresis, the milk blocking solution was sealed for approximately 1 hour, the primary antibodies were incubated, and the protein was then placed in a 4┬░C refrigerator (12ŌĆō18 hours). The primary antibodies used were anti-Bcl-2-associated X protein (Bax) (ab32503, 1/1,000), anti-cleaved-caspase-3 (ab32042, 1/500), anti-B-cell lymphoma-2 (ab32124, 1/1,000), anti-AKT (ab233755, 1/1,000), anti-p-AKT (phospho T308; ab38449, 1/1,000), anti-FOXO3 (ab23683, 1 ┬Ąg/mL), anti-p-FOXO3 (phospho S253; ab47285, 1/1,000), and anti-glyceraldehyde 3-phosphate dehydrogenase (all from Abcam, Boston MA, USA). The secondary antibody was added and incubated at room temperature in a shaker for 1 hour. The i-Bright FL1000 gel imaging system was then used for development and fixing.
The removed kidney tissues were homogenized and centrifuged to obtain the supernatant, which was used for detecting 8-hydroxy-2ŌĆÖ-deoxyguanosine (8-OHdG) (AB5830; Sigma) and 4-hydroxy-2-hexenal (4-HHE) (IDK-AA1010.1; AmyJet, Wuhan, Hubei, China) by Enzyme-Linked Immunosorbent Assay (ELISA) methods. Malondialdehyde (MAK085; Sigma), glutathione (MAK440; Sigma), catalase (CAT100; Sigma), glutathione S-transferase (MAK453; Sigma), and glutathione reductase (Sigma) levels were detected using the corresponding kits. Blood urea nitrogen, serum creatinine, and glucose levels in peripheral blood samples were measured using a quantitative enzyme colorimetric method (Dri-chem 4000i; Fujifilm, Tokyo, Japan). Hemoglobin A1c (HbA1c) levels in the red cell lysates were determined using high-performance liquid chromatography (Bio-Rad, Richmond, CA, USA).
The kidney tissue was fixed, followed by conventional paraffin-embedded sectioning, dewaxing, and dehydration. Histopathological evaluation was performed with hematoxylin and eosin (H&E) staining. According to the percentage of damaged tubules among the total tubules, the H&E injury score of renal tubules was as follows: 0 points for no lesion, 1 point for 25%, 2 points for 26ŌĆō50%, 3 points for 51ŌĆō74%, and 4 points for 75%.
The TUNEL in situ detection kit (Roche, Shanghai, China) was used to detect apoptosis in renal tubular epithelial cells. The nuclei were observed under an optical microscope according to the manufacturerŌĆÖs instructions. The normal nuclei were blue, while the brown stained cells were apoptotic cells with positive TUNEL staining. Images were analyzed and processed using ImageJ software (NIH, Bethesda, MA, USA), and five non-overlapping fields were randomly selected for each image.
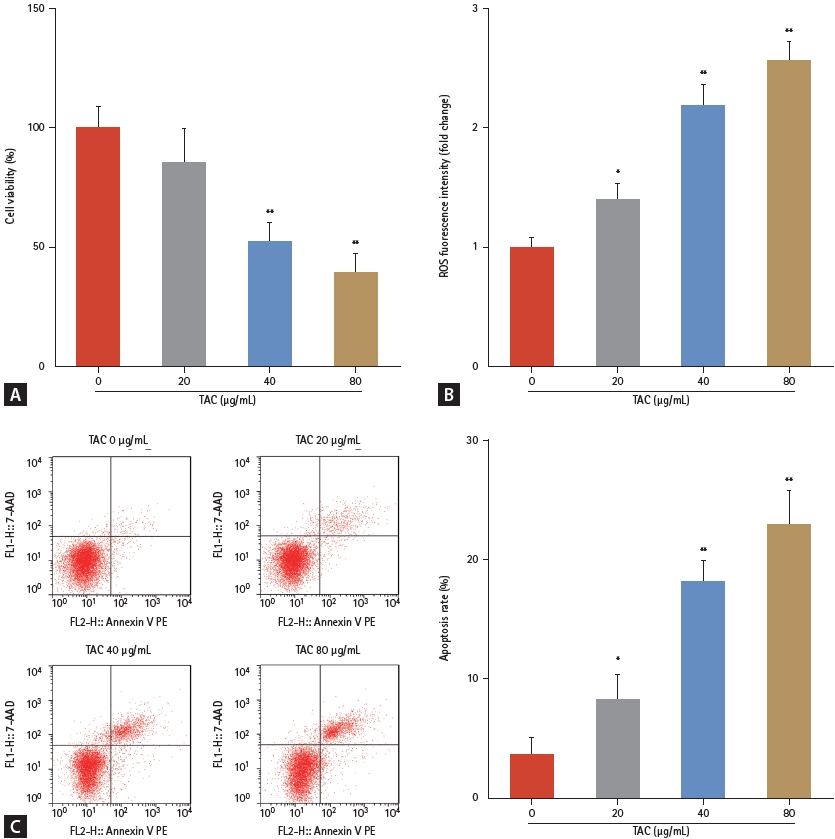
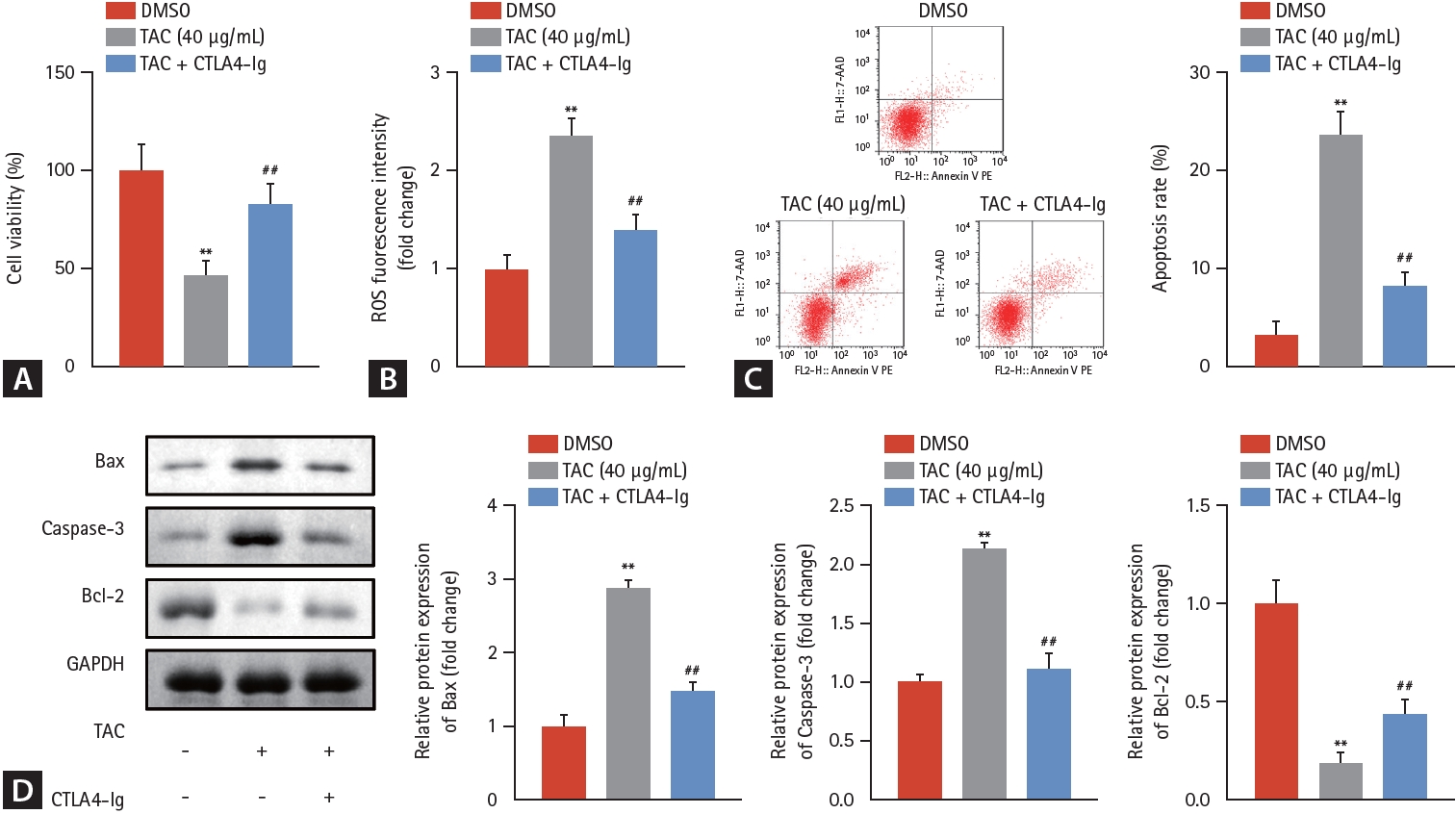
First, the effects of TAC on HK-2 cellular functions were studied. Compared with the 0 ╬╝g/mL TAC group, TAC doses Ōēź 40 ╬╝g/mL significantly suppressed cell viability (Fig. 1A), increased ROS fluorescence intensity (Fig. 1B), and accelerated apoptosis (Fig. 1C) of HK-2 cells in a dose-dependent manner. A TAC concentration of 40 ╬╝g/mL was selected for subsequent experiments. After eliminating the interference of dimethyl sulfoxide, our data revealed that CTLA4-Ig prominently promoted cell viability (Fig. 2A), decreased ROS fluorescence intensity (Fig. 2B), and decelerated apoptosis (Fig. 2C, D) of HK-2 cells, demonstrating the antagonism of CTLA4-Ig in inhibiting HK-2 cell growth induced by TAC.
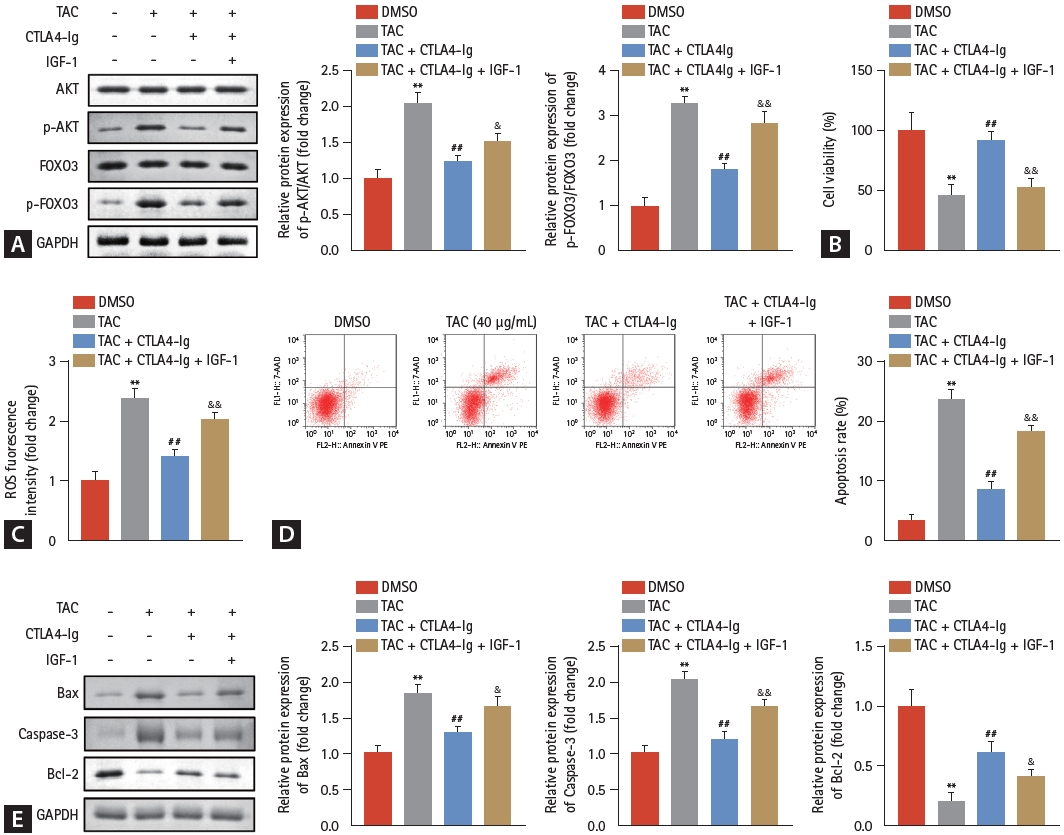
Subsequently, the activation of AKT/FOXO3 pathway was evaluated, and the results demonstrated that phosphorylated AKT and FOXO3 elevated by TAC were significantly suppressed by CTLA4-Ig (Fig. 3A), illustrating that CTLA4-Ig may protect HK-2 cells from TAC-induced damage by inactivating the AKT/FOXO3 pathway. The AKT/FOXO3 pathway was activated by IGF-1 (an agonist for PI3K/AKT) for reverse validation, and as we speculated, activation of the AKT/FOXO3 pathway prominently restrained the effects of CTLA4-Ig on cell viability (Fig. 3B), ROS intensity (Fig. 3C), and apoptosis of HK-2 cells (Fig. 3D). We found that the promoting effect of TAC and the antagonizing effect of CTLA4-Ig on apoptosis were also manifested in the regulation of apoptosis-related proteins (Fig. 3E).
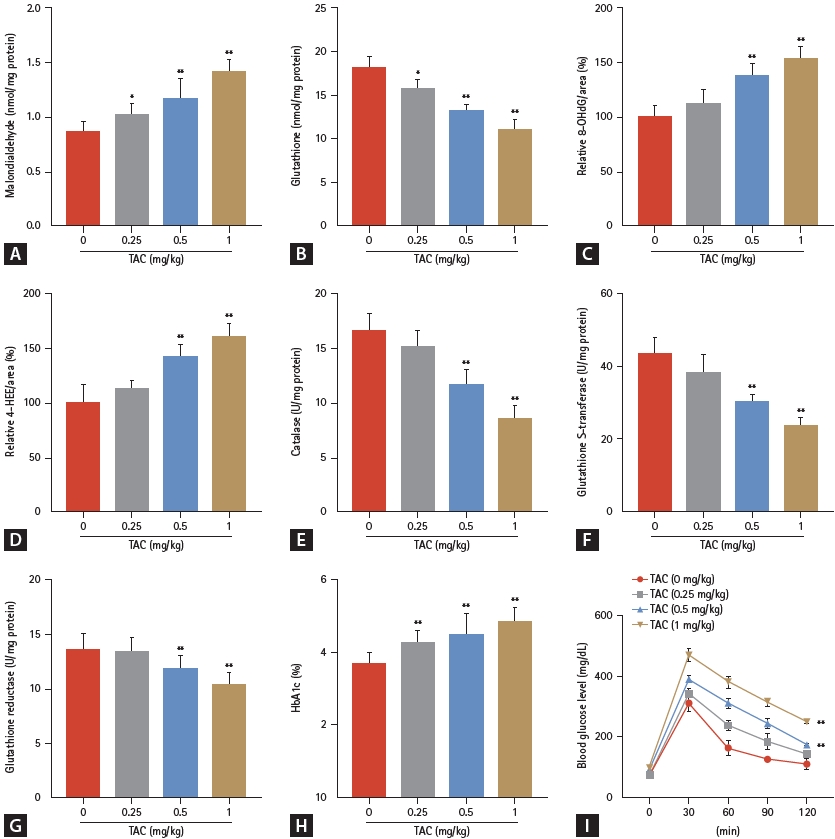
Next, we investigated whether CTLA4-Ig protects the kidney from TAC-induced damage in vivo. First, H&E staining revealed that with an increase in TAC concentration, the renal tissue of rats was damaged, which manifested as vacuolar or granular degeneration of renal tubular epithelial cells, cell flattening, lumen dilation, brush edge shedding, even bare basement membrane, and tubular formation (Fig. 4A). Meanwhile, TAC concentrations over 0 mg/kg significantly increased the H&E injury score and indicators of renal function. TAC prominently increased the H&E injury score (Fig. 4B), blood urea nitrogen (Fig. 4C), and creatinine (Fig. 4D) in a dose-dependent manner. Collectively, TUNEL staining showed that the number of apoptotic renal tubular epithelial cells increased with increasing TAC concentration (Fig. 4E, F). Furthermore, oxidative stress indicators, including malondialdehyde, 8-OHdG, and 4-HHE levels, were all significantly elevated by TAC over 0 mg/kg, while glutathione, catalase, glutathione S-transferase, and glutathione reductase levels were prominently decreased in a dose-dependent manner (Fig. 5A-G). Studies have shown that TAC may cause diabetes and thereby affect kidney function. Our data also suggest that HbA1c and blood glucose levels in rats were induced by TAC (Fig. 5H, I).
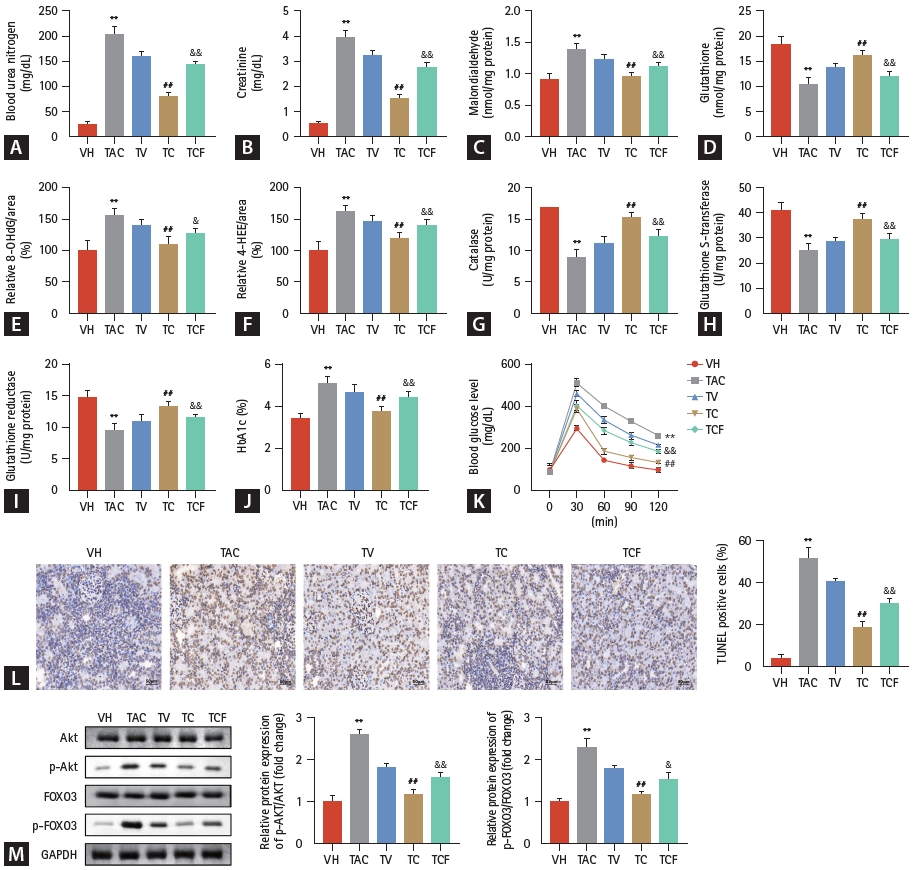
TAC at a concentration of 1.0 mg/kg was selected for subsequent studies. As we speculated, compared with the TV group, CTLA4-Ig treatment significantly reversed renal function indices induced by TAC, including blood urea nitrogen, creatinine, malondialdehyde, glutathione, 8-OHdG, 4-HHE, catalase, glutathione S-transferase, glutathione reductase, HbA1c, and blood glucose levels (Figs. 6A-K). Furthermore, the number of stained renal tubular epithelial cells evaluated by TUNEL assay was also downregulated by CTLA4-Ig compared with the TV group (Fig. 6L). This result was consistent with the trend of the cell experiment results. Meanwhile, phosphorylation of the AKT/FOXO3 pathway was also elevated by TAC and could be refrained by CTLA4-Ig in vivo (Fig. 6M). The AKT/FOXO3 pathway was activated by IGF-1 for reverse validation, and as we speculated, activation of the AKT/FOXO3 pathway prominently restrained the effects of CTLA4-Ig in modulating kidney injury.
The results of this study clearly demonstrate that CTLA4-Ig improves TAC-induced renal dysfunction and interstitial fibrosis by reducing oxidative stress in vivo and in vitro, and this effect is associated with the inhibition of AKT/FOXO3 pathway. This finding suggests that CTLA4-Ig has a direct effect on protecting TAC-induced renal injury. Our study showed that TAC causes prominent renal damage, which is in accordance with previous studies [20-22]. CTLA4-Ig, however, protected against renal injury and oxidative stress from TAC stimulation, indicating that CTLA4-Ig can protect the kidneys from TAC-induced damage in vivo.
Chronic TAC kidney injury is associated with multiple factors, but oxidative stress is regarded as a common mechanism of TAC kidney injury, and apoptotic cell death is closely related to TAC-induced oxidative stress [23,24]. Oxidative stress in cells is often accompanied by abnormal accumulation of ROS [25]. Abnormally increased ROS can have various toxic effects on tubule epithelial cells and even cause autophagy and apoptosis [26,27]. In the current work, we first verified the oxidative stress of TAC on normal kidney cells and found that TAC markedly induced oxidative stress and apoptotic cell death in HK-2 cells, which was in line with the observations of previous studies [28-30]. Then, we tested whether CTLA4-Ig has a direct protective effect against TAC-induced renal tubular injury and found that CTLA4-Ig markedly suppressed oxidative stress and decreased apoptotic cell death of TAC-treated HK-2 cells, indicating that CTLA4-Ig directly protects against TAC-induced renal tubular damage by decreasing oxidative stress.
Next, we evaluated whether this decrease in oxidative stress is related to the inactivation of AKT/FOXO3 pathway. The PI3K/AKT signaling pathway regulates FOXO through phosphorylation. AKT-mediated phosphorylation of FOXO inhibits FOXO activity by promoting its interaction with 14-3-3 proteins and nuclear exportation, and by inducing its degradation by proteasomes [31]. FOXO3a can upregulate manganese superoxide dismutase (MnSOD) expression [32-34] and thereby function as a negative regulator of mitochondrial ROS production [35] and is closely associated with resistance to oxidative stress. We previously reported that in an experimental model of TAC-induced nephropathy, TAC inhibited PI3K/AKT-mediated phosphorylation of FOXO3a and decreased FOXO3a binding to the MnSOD promoter. Thus, TAC causes mitochondrial dysfunction and increases ROS production [36]. As a direct downstream signaling molecule of the PI3K/AKT signaling pathway, FOXO3 phosphorylation is regulated by the PI3K/AKT signaling pathway [37]. It has been suggested that the PI3K/AKT/FOXO3 signaling pathway can regulate various pathological processes related to diabetic nephropathy, such as apoptosis of podocytes, glomerular stromal hyperplasia, and renal tubular epithelial cell fibrosis [38,39]. CTLA4-Ig has been reported to regulate the oxidative stress behavior of dendritic cells in non-obese diabetic mice by activating FOXO3 [40]. In this study, we found that the AKT/FOXO3 pathway in HK-2 cells was activated after TAC treatment and this activation was suppressed by CTLA4-Ig. These data indicate that CTLA4-Ig may exert its protective function against renal tubular cell damage caused by TAC by inhibiting the activation of the AKT/FOXO3 signaling pathway. To verify our hypothesis, HK-2 cells were treated with IGF-1, an agonist of this pathway, and as expected, IGF-1 activation of AKT/FOXO3 signaling significantly reversed the protective effect of CTLA4-Ig in vitro.
According to cell experiments, CTLA4-Ig can play a role in inhibiting TAC-induced injury of HK-2 cells, and this protective effect was further studied in vivo. Whether TAC causes renal injury in experimental rats was evaluated by blood urea nitrogen, creatinine, inflammatory infiltration of renal tubular epithelial cells, and the degree of apoptosis. The results revealed that the renal tissue of rats was damaged and the apoptosis of renal tubular epithelial cells was promoted with an increase in TAC concentration. TAC prominently increased blood urea nitrogen and creatinine levels in a dose-dependent manner. Collectively, TAC induced renal injury in rats, as expected.
Whether TAC can induce oxidative stress and renal injury in vivo was further investigated. Malondialdehyde is a highly toxic marker of lipid peroxidation [41], 8-OHdG is a biomarker of oxidative DNA damage [42], and 4-HHE, which accumulates in patients with chronic kidney disease [43], is also associated with oxidative stress [44]. These three indicators were prominently increased in the renal tissues of rats treated with TAC, indicating that TAC induced oxidative damage in vivo. Meanwhile, glutathione plays a pivotal role in protecting cells against oxidative stress-induced cellular damage [45], and glutathione reductase is responsible for maintaining the supply of reduced glutathione [46]. Catalase and glutathione S-transferase are enzymes involved in oxidative stress detoxification [47,48]. These four indicators were significantly decreased by TAC in a dose-dependent manner, suggesting that oxidative stress was induced by TAC. Collectively, TAC induced oxidative stress in rats with renal injury.
Hence, we speculated that inhibition of TAC-induced oxidative stress may contribute to the remission of renal injury. CTLA4-Ig treatment prominently reversed renal function indices induced by TAC, including blood urea nitrogen, creatinine, malondialdehyde, glutathione, 8-OHdG, 4-HHE, catalase, glutathione S-transferase, and glutathione reductase levels. Furthermore, the number of apoptotic renal tubular epithelial cells was downregulated by CTLA4-Ig. This observation was consistent with the results of cell experiments that showed that CTLA4-Ig inhibited TAC-induced oxidative stress to remit renal injury.
FOXO3 can reduce damage to the filter barrier and apoptosis caused by the shedding of renal podocytes from the basement membrane due to abnormal glucose metabolism, and it can stimulate the transforming growth factor beta/Smad pathway to inhibit the occurrence of renal fibrosis in renal tissue [49]. Therefore, AKT/FOXO3 may play a key regulatory role in renal injury in vivo. Inactivation of the AKT/FOXO3 pathway by CTLA4-Ig significantly alleviated kidney injury induced by TAC in vitro. Rats in the TAC group showed typical histological features of chronic kidney disease after 6 weeks, similar to those observed in human kidney tissue. Likewise, inactivation of the AKT/FOXO3 pathway also helped remit kidney injury in vivo. To verify the protective effect of CTLA4-Ig on renal damage caused by TAC through inhibiting the activation of the AKT/FOXO3 signaling pathway, rats were treated with IGF-1, which is an agonist of the AKT/FOXO3 signaling pathway [16]. Studies have shown that IGF-1 activates AKT to balance the inhibitory and excitatory signals of FOXO3 and prevent its pro-apoptotic effects during oxidative stress [50-52]. In our study, IGF-1 activation of AKT/FOXO3 signaling significantly reversed the protective effect of CTLA4-Ig in vivo, which was specifically shown to regulate renal injury indicators, including blood urea nitrogen and creatinine. IGF-1 also reversed the regulatory effect of CTLA4-Ig in alleviating oxidative stress by activating the AKT/FOXO3 pathway.
The results of our experimental study can be translated into clinical practice. Until now, CTLA4-Ig has been considered to replace calcineurin inhibitors, but its effect on TAC-induced organ injury has not been fully studied. Through this study, we found that CTLA4-Ig has a direct protective effect on TAC-induced renal injury, and that conversion to CTLA4-Ig effectively improved renal function and histopathology compared to TAC alone. These findings suggest that CTLA4 may provide beneficial effects in improving graft function in patients with preexisting TAC toxicity or TAC withdrawal.
CTLA4-Ig has a protective effect against renal injury induced by TAC, and its mechanism may be related to improving renal fibrosis, regulating apoptosis, and inhibiting oxidative stress through the AKT/FOXO3 pathway.
1. Cytotoxic T-lymphocyte-associated antigen 4-immunoglobulin (CTLA4-Ig) alleviates tacrolimus (TAC)-induced oxidative stress and apoptotic cell death in human kidney 2 (HK-2) cells.
2. The protective effects of CTLA4-Ig on HK-2 cells are associated with the activation of the protein kinase B (AKT)/forkhead transcription factor (FOXO) 3 pathway.
3. CTLA4-Ig can protect the kidney from TAC-induced injury in rats through the AKT/FOXO3 pathway.
Notes
CRedit authorship contributions
Long Jin: conceptualization, investigation, writing - original draft, writing - review & editing; Nan Shen: data curation, formal analysis, writing - review & editing; Xinyu Wen: methodology, visualization, writing - review & editing; Weidong Wang: data curation, project administration, writing - review & editing; Sun Woo Lim: data curation, formal analysis, methodology, writing - review & editing; Chul Woo Yang: conceptualization, investigation, project administration, writing - review & editing
Figure┬Ā1.
TAC suppressed (A) cell viability, increased (B) ROS fluorescence intensity, and accelerated (C) cell apoptosis of human kidney 2 cells in a dose-dependent manner. TAC, tacrolimus; ROS, reactive oxygen species; 7-AAD, 7-aminoactinomycin D. *p < 0.05, **p < 0.01 (vs. 0 ╬╝g/mL TAC).
Figure┬Ā2.
CTLA4-Ig prominently promoted (A) cell viability, decreased (B) ROS fluorescence intensity, and decelerated (C, D) cell apoptosis of human kidney 2 cells. DMSO, dimethyl sulfoxide; TAC, tacrolimus; CTLA4-Ig, cytotoxic T-lymphocyte-associated antigen 4-immunoglobulin; ROS, reactive oxygen species; Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma-2; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. **p < 0.01 (vs. DMSO), ##p < 0.01 (vs. TAC).
Figure┬Ā3.
CTLA4-Ig exerts protection function on HK-2 cells by activating the AKT/FOXO3 pathway. Protein brands and quantitative graphs of (A) AKT/FOXO3 signaling proteins under treatment with TAC and CTLA4-Ig. (B) Cell viability, (C) ROS fluorescence intensity, and (D, E) cell apoptosis of HK-2 cells were assessed after IGF-1 treatment. TAC, tacrolimus; CTLA4-Ig, cytotoxic T-lymphocyte-associated antigen 4-immunoglobulin; IGF-1, insulin-like growth factor 1; AKT, protein kinase B; FOXO3, forkhead transcription factor 3; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; DMSO, dimethyl sulfoxide; ROS, reactive oxygen species; Bax, Bcl-2-associated X protein; Bcl-2, B-cell lymphoma-2; HK-2, human kidney 2. **p < 0.01 (vs. DMSO), ##p < 0.01 (vs. TAC), &p < 0.05, &&p < 0.01 (vs. TAC + CTLA4-Ig).
Figure┬Ā4.
TAC treatment induced kidney injury in rats. (A) Pathological changes and (B) injury score of renal tissue under treatment with TAC in each group (H&E staining of the renal tissue, ├Ś400). TAC prominently increased (C) blood urea nitrogen and (D) creatinine levels. (E, F) Apoptosis of renal tubule epithelial cells in each group (TUNEL staining of the renal tissue, ├Ś200). TAC, tacrolimus; H&E, hematoxylin and eosin; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling. *p < 0.05, **p < 0.01 (vs. 0 mg/kg TAC).

Figure┬Ā5.
TAC treatment induced oxidative stress in rats. (A) Malondialdehyde, (C) 8-OHdG, and (D) 4-HHE levels were elevated while (B) glutathione, (E) catalase, (F) glutathione S-transferase, and (G) glutathione reductase levels were prominently decreased by TAC treatment in rats. (H) HbA1c and (I) blood glucose levels were evaluated under treatment with TAC. TAC, tacrolimus; 8-OHdG, 8-hydroxy-2ŌĆÖ-deoxyguanosine; 4-HHE, 4-hydroxy-2-hexenal; HbA1c, hemoglobin A1. *p < 0.05, **p < 0.01 (vs. 0 mg/kg TAC).
Figure┬Ā6.
AKT/FOXO3 pathway activated by CTLA4-Ig participates in renal injury impairment induced by TAC in vivo. (A-I) Indicators of renal injury. (J) HbA1c and (K) blood glucose levels were evaluated under different treatments. (L) Apoptosis of renal tubule epithelial cells in each group assessed using TUNEL assay. (M) Protein bands of AKT/FOXO3 pathway. VH, vehicle; TAC, tacrolimus; TV, conversion from TAC to olive oil; TC, Conversion from TAC to 1 mg/kg of CTLA4-Ig; CTLA4-Ig, cytotoxic T-lymphocyte-associated antigen 4-immunoglobulin; TCF, conversion from TAC to 1 mg/kg of CTLA4-Ig along withinsulin-like growth factor 1; 8-OHdG, 8-hydroxy-2ŌĆÖ-deoxyguanosine; 4-HHE, 4-hydroxy-2-hexenal; HbA1c, hemoglobin A1; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling; AKT, protein kinase B; FOXO3, forkhead transcription factor 3; GAPDH, glyceraldehyde 3-phosphate dehydrogenase. **p < 0.01 (vs. VH), ##p < 0.01 (vs. TAC), &p < 0.05, &&p < 0.01 (vs. TC).
REFERENCES
1. Li L, Sun Q. Renal transplantation in China: ten years of experience at Nanjing Jinling Hospital. In: Clinical transplants 2006. Los Angeles (CA): Terasaki Research Institute, 2006.
2. Tomasoni S, Remuzzi G, Benigni A. Allograft rejection: acute and chronic studies. Contrib Nephrol 2008;159:122ŌĆō134.


3. Miano TA, Flesch JD, Feng R, et al. Early tacrolimus concentrations after lung transplant are predicted by combined clinical and genetic factors and associated with acute kidney injury. Clin Pharmacol Ther 2020;107:462ŌĆō470.



4. Braithwaite HE, Darley DR, Brett J, Day RO, Carland JE. Identifying the association between tacrolimus exposure and toxicity in heart and lung transplant recipients: a systematic review. Transplant Rev (Orlando) 2021;35:100610.


5. Lim SW, Shin YJ, Luo K, et al. Effect of Klotho on autophagy clearance in tacrolimus-induced renal injury. FASEB J 2019;33:2694ŌĆō2706.



6. Lim SW, Jin L, Piao SG, Chung BH, Yang CW. Inhibition of dipeptidyl peptidase IV protects tacrolimus-induced kidney injury. Lab Invest 2015;95:1174ŌĆō1185.



7. Lee D, Lee DS, Jung K, et al. Protective effect of ginsenoside Rb1 against tacrolimus-induced apoptosis in renal proximal tubular LLC-PK1 cells. J Ginseng Res 2018;42:75ŌĆō80.



8. Greenfield EA, Nguyen KA, Kuchroo VK. CD28/B7 costimulation: a review. Crit Rev Immunol 1998;18:389ŌĆō418.


9. Pilat N, Mahr B, Gattringer M, Baranyi U, Wekerle T. CTLA4Ig improves murine iTreg induction via TGF╬▓ and suppressor function in vitro. J Immunol Res 2018;2018:2484825.




10. Jaiswal SR, Bhakuni P, Aiyer HM, Soni M, Bansal S, Chakrabarti S. CTLA4Ig in an extended schedule along with sirolimus improves outcome with a distinct pattern of immune reconstitution following post-transplantation cyclophosphamide-based haploidentical transplantation for hemoglobinopathies. Biol Blood Marrow Transplant 2020;26:1469ŌĆō1476.


11. Kumar D, LeCorchick S, Gupta G. Belatacept as an alternative to calcineurin inhibitors in patients with solid organ transplants. Front Med (Lausanne) 2017;4:60.



12. Jin L, Lim SW, Jin J, et al. Effect of conversion to CTLA4Ig on tacrolimus-induced diabetic rats. Transplantation 2018;102:e137ŌĆōe146.


13. Wang Y, Lin Y, Wang L, et al. TREM2 ameliorates neuroinflammatory response and cognitive impairment via PI3K/AKT/ FoxO3a signaling pathway in AlzheimerŌĆÖs disease mice. Aging (Albany NY) 2020;12:20862ŌĆō20879.


14. Zhang X, Wang L, Peng L, et al. Dihydromyricetin protects HUVECs of oxidative damage induced by sodium nitroprusside through activating PI3K/Akt/FoxO3a signalling pathway. J Cell Mol Med 2019;23:4829ŌĆō4838.




15. Yoon HE, Kim SJ, Kim SJ, Chung S, Shin SJ. Tempol attenuates renal fibrosis in mice with unilateral ureteral obstruction: the role of PI3K-Akt-FoxO3a signaling. J Korean Med Sci 2014;29:230ŌĆō237.




16. Zhao S, Wang L, Zhang C, et al. Inhibitor of growth 3 induces cell death by regulating cell proliferation, apoptosis and cell cycle arrest by blocking the PI3K/AKT pathway. Cancer Gene Ther 2018;25:240ŌĆō247.



17. Jin L, Lim SW, Doh KC, et al. Dipeptidyl peptidase IV inhibitor MK-0626 attenuates pancreatic islet injury in tacrolimus-induced diabetic rats. PLoS One 2014;9:e100798.



18. Chevalier RL, Goyal S, Kim A, Chang AY, Landau D, LeRoith D. Renal tubulointerstitial injury from ureteral obstruction in the neonatal rat is attenuated by IGF-1. Kidney Int 2000;57:882ŌĆō890.


19. Haffner D, Grund A, Leifheit-Nestler M. Renal effects of growth hormone in health and in kidney disease. Pediatr Nephrol 2021;36:2511ŌĆō2530.




20. Fu R, Tajima S, Shigematsu T, et al. Establishment of an experimental rat model of tacrolimus-induced kidney injury accompanied by interstitial fibrosis. Toxicol Lett 2021;341:43ŌĆō50.


21. Jiang YJ, Cui S, Luo K, et al. Nicotine exacerbates tacrolimus-induced renal injury by programmed cell death. Korean J Intern Med 2021;36:1437ŌĆō1449.




22. Jin J, Jin L, Luo K, Lim SW, Chung BH, Yang CW. Effect of empagliflozin on tacrolimus-induced pancreas islet dysfunction and renal injury. Am J Transplant 2017;17:2601ŌĆō2616.



23. Yang CC, Sung PH, Chiang JY, et al. Combined tacrolimus and melatonin effectively protected kidney against acute ischemia-reperfusion injury. FASEB J 2021;35:e21661.



24. Piao SG, Lim SW, Doh KC, et al. Combined treatment of tacrolimus and everolimus increases oxidative stress by pharmacological interactions. Transplantation 2014;98:22ŌĆō28.


25. Chen Y, Feng X, Hu X, et al. Dexmedetomidine ameliorates acute stress-induced kidney injury by attenuating oxidative stress and apoptosis through inhibition of the ROS/JNK signaling pathway. Oxid Med Cell Longev 2018;2018:4035310.




26. Garc├Ła-P├®rez E, Ryu D, Kim HY, Kim HD, Lee HJ. Human proximal tubule epithelial cells (HK-2) as a sensitive in vitro system for ochratoxin a induced oxidative stress. Toxins (Basel) 2021;13:787.



27. Wang YL, Lee YH, Hsu YH, et al. The kidney-related effects of polystyrene microplastics on human kidney proximal tubular epithelial cells HK-2 and male C57BL/6 mice. Environ Health Perspect 2021;129:57003.



28. Gao P, Du X, Liu L, et al. Astragaloside IV alleviates tacrolimus-induced chronic nephrotoxicity via p62-Keap1-Nrf2 pathway. Front Pharmacol 2021;11:610102.



29. Zheng HL, Zhang HY, Zhu CL, et al. L-Carnitine protects against tacrolimus-induced renal injury by attenuating programmed cell death via PI3K/AKT/PTEN signaling. Acta Pharmacol Sin 2021;42:77ŌĆō87.




30. Guerrieri D, Ambrosi NG, Romeo H, et al. Secretory leukocyte proteinase inhibitor protects acute kidney injury through immune and non-immune pathways. Shock 2021;56:1019ŌĆō1027.


31. Tzivion G, Dobson M, Ramakrishnan G. FoxO transcription factors; regulation by AKT and 14-3-3 proteins. Biochim Biophys Acta 2011;1813:1938ŌĆō1945.


32. Kurosu H, Yamamoto M, Clark JD, et al. Suppression of aging in mice by the hormone Klotho. Science 2005;309:1829ŌĆō1833.



33. Yamamoto M, Clark JD, Pastor JV, et al. Regulation of oxidative stress by the anti-aging hormone klotho. J Biol Chem 2005;280:38029ŌĆō38034.



35. Emerling BM, Weinberg F, Liu JL, Mak TW, Chandel NS. PTEN regulates p300-dependent hypoxia-inducible factor 1 transcriptional activity through forkhead transcription factor 3a (FOXO3a). Proc Natl Acad Sci U S A 2008;105:2622ŌĆō2627.



36. Lim SW, Jin L, Luo K, et al. Klotho enhances FoxO3-mediated manganese superoxide dismutase expression by negatively regulating PI3K/AKT pathway during tacrolimus-induced oxidative stress. Cell Death Dis 2017;8:e2972.


37. Reyes HD, Carlson MJ, Devor EJ, et al. Downregulation of FOXO1 mRNA levels predicts treatment failure in patients with endometrial pathology conservatively managed with progestin-containing intrauterine devices. Gynecol Oncol 2016;140:152ŌĆō160.



38. Du M, Wang Q, Li W, et al. Overexpression of FOXO1 ameliorates the podocyte epithelial-mesenchymal transition induced by high glucose in vitro and in vivo. Biochem Biophys Res Commun 2016;471:416ŌĆō422.


39. Li W, Wang Q, Du M, et al. Effects of overexpressing FoxO1 on apoptosis in glomeruli of diabetic mice and in podocytes cultured in high glucose medium. Biochem Biophys Res Commun 2016;478:612ŌĆō617.


40. Fallarino F, Bianchi R, Orabona C, et al. CTLA-4-Ig activates forkhead transcription factors and protects dendritic cells from oxidative stress in nonobese diabetic mice. J Exp Med 2004;200:1051ŌĆō1062.




41. Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis 2005;15:316ŌĆō328.


42. Graille M, Wild P, Sauvain JJ, Hemmendinger M, Guseva Canu I, Hopf NB. Urinary 8-OHdG as a biomarker for oxidative stress: a systematic literature review and meta-analysis. Int J Mol Sci 2020;21:3743.



43. Soulage CO, Pelletier CC, Florens N, et al. Two toxic lipid aldehydes, 4-hydroxy-2-hexenal (4-HHE) and 4-hydroxy-2-nonenal (4-HNE), accumulate in patients with chronic kidney disease. Toxins (Basel) 2020;12:567.



44. Saeki T, Ichiba M, Tanabe N, et al. Expression of oxidative stress-related molecules in circulating leukocytes and urine in patients with chronic viral hepatitis. Liver Int 2006;26:157ŌĆō165.


45. Homma T, Fujii J. Application of glutathione as anti-oxidative and anti-aging drugs. Curr Drug Metab 2015;16:560ŌĆō571.

46. Couto N, Wood J, Barber J. The role of glutathione reductase and related enzymes on cellular redox homoeostasis network. Free Radic Biol Med 2016;95:27ŌĆō42.


47. Kim JL, Reader BF, Dumond C, et al. Pegylated-catalase is protective in lung ischemic injury and oxidative stress. Ann Thorac Surg 2021;111:1019ŌĆō1027.


48. Tahir M, Rehman MYA, Malik RN. Heavy metal-associated oxidative stress and glutathione S-transferase polymorphisms among E-waste workers in Pakistan. Environ Geochem Health 2021;43:4441ŌĆō4458.



49. Qin G, Zhou Y, Guo F, et al. Overexpression of the FoxO1 ameliorates mesangial cell dysfunction in male diabetic rats. Mol Endocrinol 2015;29:1080ŌĆō1091.



50. D├Īvila D, Torres-Aleman I. Neuronal death by oxidative stress involves activation of FOXO3 through a two-arm pathway that activates stress kinases and attenuates insulin-like growth factor I signaling. Mol Biol Cell 2008;19:2014ŌĆō2025.



- TOOLS




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement figure 1
Supplement figure 1 Print
Print



