 |
 |
| Korean J Intern Med > Volume 38(5); 2023 > Article |
|
Abstract
Background/Aims
The overall incidence of pneumococcal pneumonia is declining. However, the change in the pathogenic distribution of community-acquired pneumonia (CAP) in chronic obstructive pulmonary disease (COPD) patients and the serotype specificity of Streptococcus pneumoniae have not been evaluated in the post-era of pneumococcal vaccination in Korea.
Methods
We conducted a prospective, multi-center, cohort study from seven University-affiliated hospitals. The primary objective was the identification of serotype-specific prevalence of pneumococcal pneumonia in COPD patients hospitalized for CAP. For the purpose, we conducted serotype-specific urine antigen detection (SS-UAD) assays for S. pneumoniae. The secondary objectives were other clinical characteristics of pneumonia including vaccination status.
Results
The total number of participants was 349. Most of them were male (95.1%) with old ages (75.55 ± 8.59 y). The positive rate for S. pneumoniae was 9.2% with SS-UAD assay and the common serotypes were 22F, 6A, and 6B. In the sputum, Pseudomonas aeruginosa (5.0%) and Haemophilus influenzae (4.0%) were common pathogens. The vaccination rate was 78.8%, 53.0%, and 25.8% for influenza, pneumococcal polysaccharide vaccine 23 (PPV 23), and pneumococcal protein-conjugated vaccine 13 (PCV 13), respectively. Thirteen patients died during hospitalization (mortality rate; 3.7%). There was no difference in the respective rate of influenza vaccination (79.2% vs. 69.2%, p = 0.288) and PCV 13 vaccination (25.6% vs. 30.8%, p = 0.443) between survivors and the deceased.
Chronic obstructive pulmonary disease (COPD) is one of the leading causes of morbidity and mortality worldwide. By 2030, COPD is expected to be the third main cause of death [1]. Community-acquired pneumonia (CAP) represents not only a frequent complication but also a deadly cause in patients with COPD [2,3]. In a recent article, authors analyzed the Korean National Health and Nutrition Examination Survey (KNHANES) data from 2007 to 2015. Patients with COPD had a higher admission rate than those without COPD and the hospitalization rate due to respiratory illnesses intensified as the grade of COPD advanced from Global Initiative for Chronic Obstructive Lung Disease (GOLD) 1 to GOLD 4 [4].
The prevalence of pathogens for pneumonia varies depending on geography, comorbidities, vaccination status, and site of care. Usually, the most commonly identified pathogens of CAP are Streptococcus pneumoniae and respiratory viruses [5–7]. It is important to identify pathogens for pneumonia in the general population and in specific, highrisk groups for decision-making of appropriate antibiotics. Unfortunately, in more than half of cases, pathogens are not detected despite extensive microbiologic testing [8,9]. In addition, the distribution of pathogens for CAP changes over time. Especially, the overall incidence of pneumonia due to S. pneumoniae is declining, in part due to widespread use of pneumococcal vaccination [6]. Furthermore, serotypes of pneumococcus that cause pneumonia change depending on the coverage of the specific serotypes in pneumococcal vaccine [10]. In Korea, the cost for pneumococcal polysaccharide vaccine 23 (PPV 23) is covered by the National Immunization Program (NIP) since May 2013, which resulted in high vaccination rate among adults. However, pneumococcal protein-conjugated vaccine 13 (PCV 13) is approved but not supported by the NIP, which explains the low rate of PCV13 vaccination among elderly people [11]. Elderly patients with COPD are one of the most high-risk populations for pneumococcal pneumonia. Introduction of PPV 23 or PCV 13, has delivered a profound impact on causative pathogens for pneumonia in that population. However, to our knowledge, the change in the pathogenic distribution of CAP in patients with COPD, not to mention of serotype specificity of S. pneumoniae have not been evaluated in the post-era of vaccinations in Korea.
We conducted a prospective, multi-center, cohort study with the collaboration of Korean pulmonologists in seven university-affiliated hospitals. We evaluated the pathogen distribution, influenza and pneumococcal vaccination status, and other clinical characteristics of hospitalized pneumonia patients with COPD. Moreover, we conducted serotype-specific urine antigen detection (SS-UAD) assay for S. pneumoniae.
The primary objective was the identification of serotype-specific prevalence of pneumococcal pneumonia in hospitalized pneumonia patients with COPD. Methods of pathogen identification were sputum Gram stain/culture and two sets of blood cultures if possible. The SS-UAD assay for pneumococcus was performed at the Pfizer Central Lab in the US in accordance with the standardized protocol [12]. The secondary objectives were to detect any differences between influenza/pneumococcal vaccine recipients and non-recipients with respect to outcomes such as mortality and intensive care unit (ICU) admission rate, and clinical characteristics of COPD patients hospitalized due to CAP including demographic data, comorbidities, vaccination status (for PCV 13, PPV 23, and influenza), and severity scores of pneumonias (pneumonia severity index [PSI] and CURB-65).
This study was a prospective, multi-center, cohort study and seven university-affiliated medical institutes participated in the study from May 2, 2017 to Feb 3, 2020. Subjects of the study were patients with COPD who were hospitalized due to CAP. The inclusion criteria for COPD were (1) male or female over 40 years; (2) current or ex-smokers with smoking history ≥ 10 pack-years; (3) post bronchodilator FEV1/ FVC ratio < 0.7; (4) no other chest radiologic abnormalities explaining the obstructive pattern in spirometry; and (5) no current diagnosis of bronchial asthma. The most recent data of pulmonary function tests were used for evaluation, which were measured before the development of pneumonia. The classification of COPD group was determined by the 2016 guideline of Global Initiative for chronic obstructive lung disease (GOLD) [13]. The inclusion criteria for CAP were newly developed pneumonic infiltrates on chest radiography with at least one of following three criteria: (1) body temperature (< 36°C or ≥ 38.0°C); (2) white blood cell count (< 5,000/mm3 or > 10,000/mm3); or (3) cough and/or sputum [5]. This study was approved by the Institutional Review Board (IRB) of each participating institution. The IRB number of the representing institution, Chung-Ang University Hospital, was CAUH 1601-001-254. Informed consent was obtained from all participants. Data collection from seven participating institutions was performed by using a web-based registration program (http://project.swu.ac.kr/copdcap).
Because the primary objective was a descriptive parameter, we used CURB-65 for the calculation of sample size. The sample size calculations were based on detection of a one-point difference in CURB-65 score between PCV 13 recipients and non-recipients (expected mean CURB-65 score of three points for PCV 13 recipients and four points for non-recipients). Common standard deviation was assumed to be one point in each group with a two-tailed test at 5% type I error, and a desired power of 80% [14]. The estimated sample size for an independent t-test was 90 when we expected that 10% of them were PCV 13 recipients and the other 90% were non-recipients (at the time of study launch, 10% of Korean adults were estimated to be vaccinated with PCV13 [11]). Because the CURB-65 scores may not be symmetrically distributed, 15% of patient numbers were added for a non-parametric test. The desired number of patients with pneumococcal pneumonia was 104 for the analysis. A minimal required number was 346 assuming 30% prevalence of pneumococcal pneumonia among CAP (the prevalence of pneumococcal pneumonia was 25–45% in Korea [15]). The total required number of CAP patients was 384 with allowance of 10% possible data loss.
Continuous variables were evaluated with analysis for non-normal distribution. Continuous variables were presented as median [interquartile range] and categorical data as frequencies and percentiles. Intergroup comparisons of continuous variables and categorical data between survivors and deceased patients were performed with Mann–Whitney tests, chi-square tests and Fisher’s exact test, respectively. Because the number of the deceased was limited, the evaluation of risk factors for mortality and ICU admission were performed with univariate logistic regression analysis. The results were analyzed using SPSS version 16.0 (SPSS Inc., Chicago, IL, USA). p values < 0.05 were considered significant.
Originally, we intended to recruit 384 patients with COPD who were hospitalized due to CAP. However, the development of the SARS-CoV2 pandemic made the recruitment of pneumonia patients very difficult at participating institutions. After reaching 92.9% of the target number (357 patients), enrollment was closed because the minimum required number of 346 subjects for analysis was met. Eight patients were additionally excluded in the final analysis; seven subjects had FEV1/FVC > 0.7 and one subject was diagnosed with pulmonary tuberculosis. A final total of 349 subjects were enrolled for the analysis.
From the total of 349 subjects, most were male (95.1%) with old ages (77.0 years of age [71.0–81.0 y]). The life-long amount of smoking was over fifty pack-year and many of them were ex-smokers (79.4%). The patients had a variety of comorbidities and hypertension was the most common condition. As was expected, all patients had post-bronchodilator FEV1/FVC less than 0.7 and percent-predicted FEV1 was 55.5% [39.0–70.0%]. The most common group of COPD severity was group B (53.9%) which was followed by group D (37.8%), group A (5.7%), and group C (2.6%). Most patients used inhaler therapy containing either long-acting beta 2 agonists (73.4%) or long-acting muscarinic antagonists (64.5%). Inhaled corticosteroids (ICSs), in combination with other long-acting bronchodilators, were utilized in 47.3% of patients (Table 1).
More than half of the subjects were hospitalized via emergency department (60.9%). Over one-tenth of them had recent hospitalization history within three months of the study. Radiologic examinations indicated unilateral infiltration in 60.8% and 16.6% of them had accompanying pleural effusion. Acute phase reactants, such as total white cell counts, percentile of neutrophil, absolute neutrophil count, and C-reactive protein were all elevated. The PSI score was 89.0 [77.0–106.5]. and many of them (87.1%) were class III or more in PSI classification. The CURB-65 score was 1.0 [1.0–2.0] for the whole population. The CURB-65 score displayed same value of 1.0 [1.0–2.0] for PCV 13 recipients and non-recipients with no statistical significance. Over two-thirds of them (74.8%) were prescribed with a combination of antibiotics. The median duration of hospitalization was 8.0 days [6.0–14.0 d]. The ICU admission rate was 4.3% and the median duration of ICU stays was 4.0 days [3.0–15.0 d]. The median cost of care for hospitalization was US$ 2,520.9 [US$ 1,427.7–4,067.9]. Most of them (91.4%) recovered from pneumonia and discharged, whereas 3.7% died (Table 2).
Sputum Gram stain and/or culture was performed for the identification of pathogens of pneumonia for 96.6% of cases. The most frequently isolated pathogen from the sputum was Pseudomonas aeruginosa (5.0%), followed by Haemophilus influenzae (4.0%), Klebsiella pneumoniae (2.3%), S. pneumoniae (1.7%), and Staphylococcus aureus (1.7%). The positive rate of the SS-UAD urine pneumococcal antigen test was 9.2%. Common serotypes were 22F, 6A, and 6B. Blood cultures were positive in 4.7% of samples and S. pneumoniae was positive in a single case (0.7%) (Table 3).
Vaccinations for seasonal influenza, PPV 23, and PCV 13 were performed in 78.8%, 53.0%, and 25.8% of subjects, respectively (Fig. 1). Dual vaccination of PPV and PCV was achieved in 10.3% of patients. The median time interval from vaccination to hospitalization was 166 days [70.5–273.8 d] for influenza vaccine, 1,981 days [1,518.3–2,405.8 d] for PPV 23, and 1,237 days [530.8–1,625.5 d] for PCV 13.
Patients with COPD who died during hospitalization due to pneumonia displayed higher values than survivors in comorbidities, vital signs, many laboratory findings including acute phase reactants, disease severity scores, duration of antibiotics treatment, duration of hospitalization, and PPV 23 vaccination. In addition, those patients who survived showed higher values in albumin and bicarbonate than deceased subjects (Table 4).
In univariate analysis, the risk of mortality increased in those who had hypertension, malignancy, and those who were administered the PPV 23 vaccination. The number of patients who received PPV23 within 3 years of hospitalization was 17 (12.1%), and 123 (87.9%) patients received PPV23 earlier than 3 years. There was no difference in ICU admission or mortality rate between the two groups. In addition, the interval from the PSV 23 vaccination to the hospitalization was longer for the deceased (n = 8, 21,850 d) than for survivors (n = 132, 1,790 d), although the difference was not statistically significant due to the small number of deaths. The risk for ICU admission increased in patients with previous pneumonia history (Table 5).
CAP is a frequent complication in COPD and once developed, it can be fatal. Pneumonia has been the third major cause of mortality for more than ten years in Korea [16]. Comorbidities such as diabetes, chronic heart diseases, cigarette smoking, chronic hepatic diseases, bronchial asthma, and COPD increase the risk for the development of pneumonia [5]. In fact, when old age (≥ 65 y) is combined with COPD, the risk for pneumococcal pneumonia increases nearly 8-fold [7]. Additionally, ICSs, which were used by nearly half of participants in the present study, escalcted the risk for pneumonia [13]. However, the burden of pneumococcal pneumonia and the serotype-specific prevalence of S. pneumoniae in patient population with COPD in the post-vaccination era have not been specifically evaluated in Korea. Therefore, we conducted a prospective, multi-center study with the collaboration of Korean pulmonologists at seven University-affiliated hospitals to evaluate pathogen distribution, and other clinical characteristics of COPD patients admitted for CAP.
The patients with COPD hospitalized for CAP were predominantly male (95.1%) with old ages (75.55 ± 8.59 y), which was expected because the smoking rate of adult Korean men, although showing a declining trend in recent decade has been high for a long period of time [17]. Participants had multiple comorbidities, with hypertension and diabetes as the most common conditions, which is consistent with other clinical studies of patients with COPD in Korea [18]. Most of patients with COPD (89.4%) were classified to either group B or group D and the percent-predicted post-bronchodilator was 55.43 ± 19.71%. In large clinical trials for COPD, patients constituting group B and group D in combination usually occupied the majority of patients [19]. In accordance with the GOLD guideline, many of participants were using inhaler therapies containing either long-acting beta 2 agonists (73.4%) or long-acting muscarinic antagonists (64.5%) with or without ICSs (Table 1). The above findings imply that the clinical profile of subjects enrolled in the present study generally represent a real world, standard feature of patients with COPD.
In the severity classification of CAP, patients with PSI class III or more are usual candidates for hospitalization [20]. This is consistent with our result that 87.1% subjects were classified to PSI class III or more (Table 2). CURB-65 score was 1.0 [1.0–2.0] for the whole population and there was no difference in CURB-65 score between PCV13 recipients and non-recipients. As for causative pathogens of pneumonia, P. aeruginosa and H. influenzae were most frequently isolated organisms from the sputum culture. When sputum culture and SS-UAD assay were combined with the consideration of 4 replicated cases, the prevalence of pneumococcal pneumonia was 9.48%. Pneumococcus usually occupies 20 to 40% of pathogens of CAP [9,11]. The reasons for a lower rate of isolation of S. pneumoniae in the present study could be related to prior, frequent antibiotic exposure before being transferred to University-affiliated hospitals of participating institutes. In the pre-PCV 13 era, 19A/F, 15A/F, 19B, and 23A were frequent serotypes of pneumococcus. Meanwhile 23A, 15A/F, and 3 were the major serotypes in the post-PCV 13 era in Korea [15]. In the present study of more specified population of COPD, common serotypes were 22F, 6A, and 6B. PPV 23 contains antigens for 22F and 6B and PCV 13 includes antigens for 6A and 6B. The frequent isolation of three serotypes covered either by PPV 23 or by PCV 13 might be explained by the prolonged interval from the time of PPV 23 inoculation to hospitalization (1,982.43 ± 795.43 d) and the low rate of vaccination for PCV 13 (25.8%) (Fig. 1). The low rate of PCV 13 vaccination is closely related to the reimbursement policy of Korean government for pneumococcal vaccination. As was noted, the costs for influenza and PPV 23 in old ages (≥ 65 y) are fully covered by NIP, but that of PCV 13 is not reimbursed.
A protective effect for mortality has not been demonstrated among recipients of influenza, PPV 23, or PCV 13 in the present study. On the contrary, deceased patients had higher rate of PPV vaccination than survivors (84.6 vs. 51.8%). It is important to note that the duration from PPV 23 vaccination to hospitalization was more than three years in most recipients (87.9%). The effect of PPV 23 vaccination wanes two to three years after inoculation, meaning that almost 90% of PPV 23 recipients in the present study would not have enough IgG titer for pneumococcal infection [21]. In addition, the interval from the PSV 23 vaccination to the hospitalization was longer for the deceased (n = 8, 21,850 d) than for survivors (n = 132, 1,790), although the difference was not statistically significant due to the small number of deaths. The increased mortality in the PPV 23 vaccination group might be related to the longer duration since vaccination. Dual vaccination with PPV 23 and PCV 13 did not differ in mortality and ICU admission rate compared to PPV 23 alone, PCV 13 alone, or non-vaccination.
There are some limitations in the present study. At first, the sample size was calculated to be 384 in the assumption that there would be a one point difference in CURB-65 score between PCV 13 recipients and non-recipients. After reaching 92.9% of target number (357 patients), we closed enrollment because of SARS-CoV2 pandemic. In addition, it was postulated that 30% of pneumonia would be caused by pneumococcus. In fact, pneumococcus explained only 9.48% of pneumonia, less than one-third of our assumption. Early closure of enrollment and low rate of pneumococcal pneumonia must have weakened the statistical power for the evaluation of vaccination effects. In addition, except for the SS-UAD test which was performed in the central lab, other radiologic and laboratory evaluation were conducted separately at each participating institution. It might have resulted in variation in the tests. Still, the present study provided valuable information regarding serotype-specific prevalence of pneumococcal pneumonia in COPD patients hospitalized for CAP in the post-vaccination era in Korea.
In summary, pneumococcus is an important pathogen for CAP developed in patients with COPD. Three serotypes, 22F, 6A, and 6B, which are covered either by PPV 23 or by PCV 13, still were prevalent serotypes of pneumococcus. The prolonged interval from PPV 23 inoculation and the low vaccination rate for PCV 13 might be plausible explanations for the noted serotypes. Further efforts should be devoted to protecting patients with COPD from preventable pneumococcal infection.
1. Pneumococcus is the most prevalent pathogen of hospitalized pneumonia in patients with COPD in post-vaccination era.
2. Three serotypes, 22F, 6A, and 6B, which are covered either by PPV 23 or by PCV 13, still were most prevalent serotypes of pneumococcus.
3. Further efforts, including the strategic augmentation of pneumococcal vaccination, need to be devoted to the prevention of pneumococcal pneumonia in COPD patients.
Notes
CRedit authorship contributions
Jae Yeol Kim: conceptualization, methodology, resources, investigation, writing - original draft, project administration, funding acquisition; Jae-Woo Jung: methodology, investigation, formal analysis, supervision; Min-Jong Kang: conceptualization, methodology, resources, validation, supervision; Deog Kyeom Kim: methodology, resources, investigation, data curation; Hayoung Choi: conceptualization, methodology, investigation, data curation, validation; Young-Jae Cho: conceptualization, methodology, investigation, data curation, visualization; Seung Hun Jang: conceptualization, methodology, resources, investigation, data curation; Chang-Hoon Lee: conceptualization, methodology, resources, investigation, validation, supervision; Yeon Mok Oh: conceptualization, methodology, resources, investigation, validation, visualization; Ji Sook Park: conceptualization, methodology, resources, data curation, formal analysis, validation, software, project administration
Figure 1
Percentage of administration of seasonal influenza vaccine, pneumococcal polysaccharide vaccine 23 (PPV 23), and pneumococcal protein-conjugated vaccine 13 (PCV 13) prior to hospitalization due to community-acquired pneumonia in patients with chronic obstructive pulmonary disease.
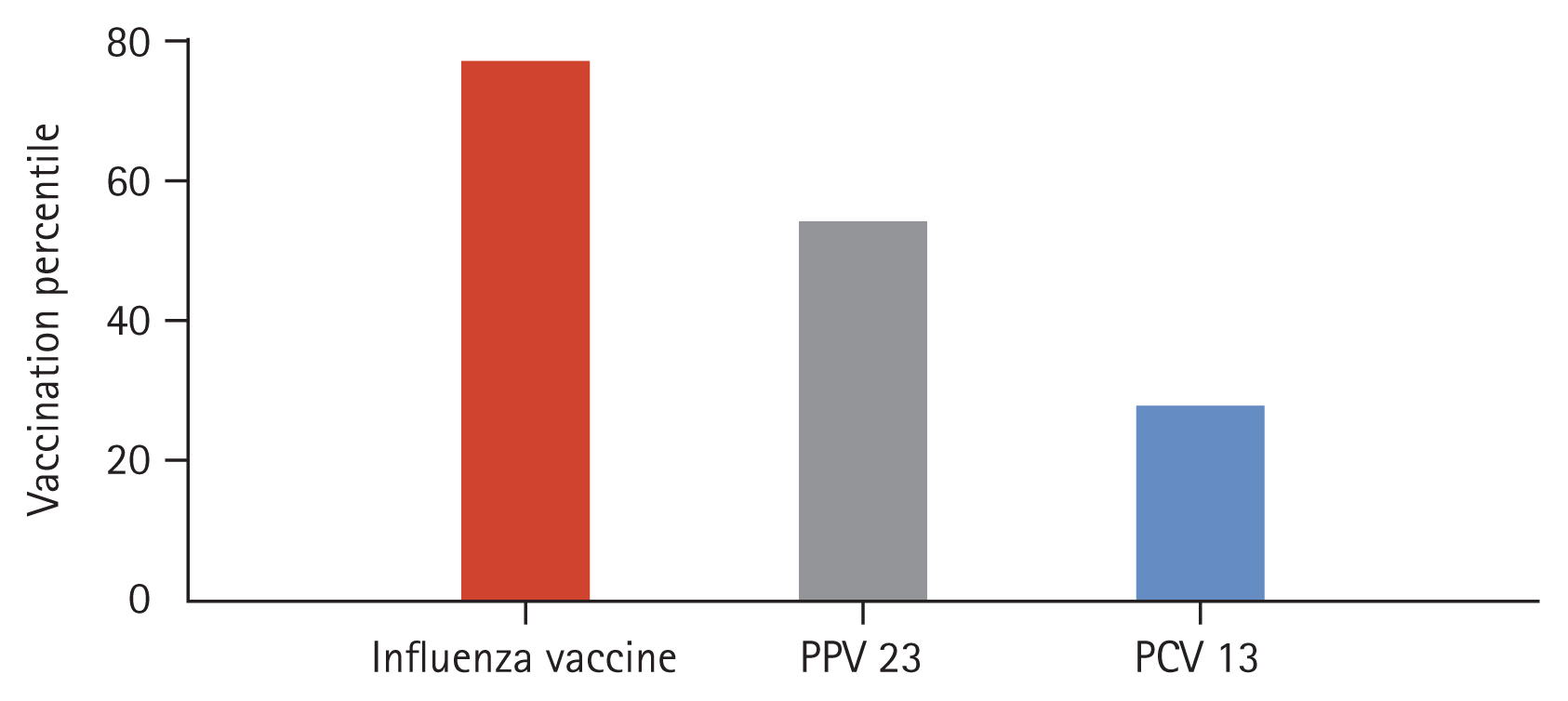
Table 1
Demographics and clinical characteristics of patients with COPD
Table 2
Clinical characteristics of community-acquired pneumonia
Table 3
Results of microbiologic examinations for causative organisms of pneumonia
Table 4
Differences in clinical characteristics between survivors and deceased patients
| Characteristic | Survivors | Deceased | p value |
|---|---|---|---|
| Number | 336 (96.3) | 13 (3.7) | |
| Age (yr) | 77.0 [71.0–81.5] | 77.0 [72.0–80.0] | 0.963 |
| Comorbidities-hypertension | 153 (45.5) | 10 (76.9) | 0.052 |
| Modified medical research council (mMRC) | 2.6 ± 1.1 | 3.2 ± 0.9 | 0.055 |
| COPD assessment test (CAT) | 25.0 [17.0–29.0] | 25.0 [23.0–34.0] | 0.223 |
| Route of admission (ER) | 200 (59.5) | 12 (92.3) | 0.037* |
| Heart rate (/min) | 93.0 [80.5–106.0] | 108.0 [98.0–119.0] | 0.007* |
| Respiratory rate(/min) | 20.0 [20.0–24.0] | 24.0 [20.0–28.0] | 0.021* |
| Percentile of neutrophils (%) | 79.0 [72.8–85.4] | 89.1 [81.1–90.5] | 0.003* |
| Absolute neutrophil count (/μL) | 7,807.5 [5,659.0–10,969.0] | 10,769.0 [9,227.0–12,927.0] | 0.009* |
| HCO3 (mmol/L) | 24.6 [22.2–27.1] | 21.6 [18.0–22.6] | 0.014* |
| Albumin (g/dL) | 3.7 [3.4–4.0] | 3.4 [3.0–3.6] | 0.009* |
| Glucose (mg/dL) | 127.0 [110.0–162.0] | 196.0 [120.0–258.0] | 0.022* |
| Pneumonia severity index score | 88.0 [77.0–105.0] | 107.0 [92.0–147.0] | 0.005* |
| CURB-65 | 1.0 [1.0–2.0] | 1.0 [1.0–2.0] | 0.231 |
| Quick sepsis related organ failure assessment | 0.0 [0.0–1.0] | 1.0 [0.0–1.0] | 0.035* |
| Days, hospitalization | 8.0 [6.0–13.0] | 27.0 [11.0–43.0] | 0.001* |
| Days, antibiotics | 10.0 [7.0–14.0] | 21.0 [9.5–30.0] | 0.004* |
| Days, systemic corticosteroids | 1.0 [0.0–7.0] | 1.0 [0.0–25.0] | 0.239 |
| Admission to ICU | 10 (3.0) | 5 (38.5) | < 0.001* |
| Days in ICU | 0.0 [0.0–0.0] | 0.0 [0.0–2.0] | < 0.001* |
| High flow oxygen | 12 (3.7) | 4 (30.8) | 0.002* |
| Non-invasive positive pressure ventilation | 2 (0.6) | 1 (8.3) | 0.105 |
| Cost per day of hospitalization (US$) | 279.3 [208.8–359.9] | 477.1 [386.7–689.4] | 0.001* |
| Polysaccharide pneumococcal vaccine | 174 (51.8) | 11 (84.6) | 0.018* |
| Protein-conjugated pneumococcal vaccine | 86 (25.6) | 4 (30.8) | 0.443 |
| Influenza vaccine | 266 (79.2) | 9 (69.2) | 0.288 |
Table 5
The univariate analysis for risk factors for mortality and ICU admission
| Characteristic | Mortality | p value | ICU admission | p value | ||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | |||
| Age | 0.988 | 0.909–1.074 | 0.777 | 0.936 | 0.869–1.009 | 0.085 |
| Body mass index | 1.001 | 0.986–1.015 | 0.920 | 0.920 | 0.803–1.053 | 0.227 |
| Alcohol | 0.862 | 0.208–3.565 | 0.837 | 2.150 | 0.572–7.950 | 0.251 |
| Smoking amount (PY) | 0.966 | 0.930–1.004 | 0.076 | 0.968 | 0.929–1.008 | 0.113 |
| Hypertension | 6.684 | 1.440–31.021 | 0.015* | 2.973 | 0.810–10.906 | 0.100 |
| Diabetes | 1.319 | 0.311–5.587 | 0.707 | 1.181 | 0.234–5.976 | 0.840 |
| Ischemic heart diseases | 0.838 | 0.139–5.064 | 0.847 | 0.997 | ||
| Chronic kidney diseases | 1.100 | 0.148–8.156 | 0.926 | 1.866 | 0.145–23.933 | 0.632 |
| Malignancies | 4.571 | 1.142–18.298 | 0.032* | 1.803 | 0.402–8.083 | 0.441 |
| Pneumonia history | 1.499 | 0.247–9.085 | 0.660 | 6.897 | 1.245–38.212 | 0.027* |
| Post-bronchodilater FEV1% | 0.979 | 0.944–1.015 | 0.252 | 0.997 | 0.965–1.030 | 0.858 |
| PPV | 10.838 | 1.788–65.708 | 0.010* | 0.472 | 0.118–1.891 | 0.289 |
| PCV | 1.289 | 0.334–4.967 | 0.713 | 1.419 | 0.400–5.030 | 0.588 |
| Influenza vaccination | 0.277 | 0.067–1.148 | 0.077 | 2.635 | 0.500–13.882 | 0.253 |
REFERENCES
1. World Health Organization. Chronic obstructive pulmonary disease (COPD) [Internet] Geneva: World Health Organization, c2023. [cited 2023 Mar 1]. Available from: https://www.who.int/news-room/fact-sheets/detail/chronic-obstructive-pulmonary-disease-(copd)
.
2. Ramirez JA, Wiemken TL, Peyrani P, et al.; University of Louisville Pneumonia Study Group. Adults hospitalized with pneumonia in the United States: incidence, epidemiology, and mortality. Clin Infect Dis 2017;65:1806–1812.


3. Rinaudo M, Ferrer M, Terraneo S, et al. Impact of COPD in the outcome of ICU-acquired pneumonia with and without previous intubation. Chest 2015;147:1530–1538.


4. Kang HK, Jung JW, Kang MJ, et al. Hospitalization increases while economic status deteriorates in late stages of chronic obstructive pulmonary disease: the Korean National Health and Nutrition Examination Survey for 2007–2015. J Thorac Dis 2021;13:2160–2168.



5. Jain S, Self WH, Wunderink RG, et al.; CDC EPIC Study Team. Community-acquired pneumonia requiring hospitalization among U.S. adults. N Engl J Med 2015;373:415–427.



6. Johansson N, Kalin M, Tiveljung-Lindell A, Giske CG, Hedlund J. Etiology of community-acquired pneumonia: increased microbiological yield with new diagnostic methods. Clin Infect Dis 2010;50:202–209.



7. Quan TP, Fawcett NJ, Wrightson JM, et al.; Infections in Oxfordshire Research Database (IORD). Increasing burden of community-acquired pneumonia leading to hospitalisation, 1998–2014. Thorax 2016;71:535–542.



8. Musher DM, Roig IL, Cazares G, Stager CE, Logan N, Safar H. Can an etiologic agent be identified in adults who are hospitalized for community-acquired pneumonia: results of a one-year study. J Infect 2013;67:11–18.



9. Musher DM, Abers MS, Bartlett JG. Evolving understanding of the causes of pneumonia in adults, with special attention to the role of pneumococcus. Clin Infect Dis 2017;65:1736–1744.



10. Isturiz R, Grant L, Gray S, et al. Expanded analysis of 20 pneumococcal serotypes associated with radiographically confirmed community-acquired pneumonia in hospitalized US adults. Clin Infect Dis 2021;73:1216–1222.




11. Heo JY, Seo YB, Choi WS, et al. Effectiveness of pneumococcal vaccination against pneumococcal pneumonia hospitalization in older adults: a prospective, test-negative study. J Infect Dis 2022;225:836–845.



12. Wunderink RG, Self WH, Anderson EJ, et al. Pneumococcal community-acquired pneumonia detected by sero-type-specific urinary antigen detection assays. Clin Infect Dis 2018;66:1504–1510.



13. Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: 2023 report [Internet] GOLD, [cited 2022 Dec 13]. Available from: www.goldcopd.org
.
14. Fernandes L, Arora AS, Mesquita AM. Role of semi-quantitative serum procalcitonin in assessing prognosis of community acquired bacterial pneumonia compared to PORT PSI, CURB-65 and CRB-65. J Clin Diagn Res 2015;9:OC01–OC044.



15. Yoo JR, Heo ST, Oh H, Oh S, Kim YR, Lee KH. Changes in serotype of Streptococcus pneumoniae after the introduction of the 13-valent pneumococcal vaccine in a homogenous population on Jeju Island. Infect Chemother 2019;51:67–72.




16. Yun S, Oh K. The Korea National Health and Nutrition Examination Survey data linked Cause of Death data. Epidemiol Health 2022;44:e2022021.




17. Choi S, Bahk J, Park S, Oh K, Jung-Choi K. Smoking, drinking, and physical activity among Korean adults before and during the COVID-19 pandemic: a special report of the 2020 Korea National Health and Nutrition Examination Survey. Epidemiol Health 2022;44:e2022043.




18. Gu KM, Yoon SW, Jung SY, et al. Acute exacerbation of COPD increases the risk of hip fractures: a nested case-control study from the Korea National Health Insurance Service. Korean J Intern Med 2022;37:631–638.







 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



