 |
 |
| Korean J Intern Med > Volume 39(1); 2024 > Article |
|
Abstract
Background/Aims
Methods
Results
Acknowledgments
Notes
Figure 1
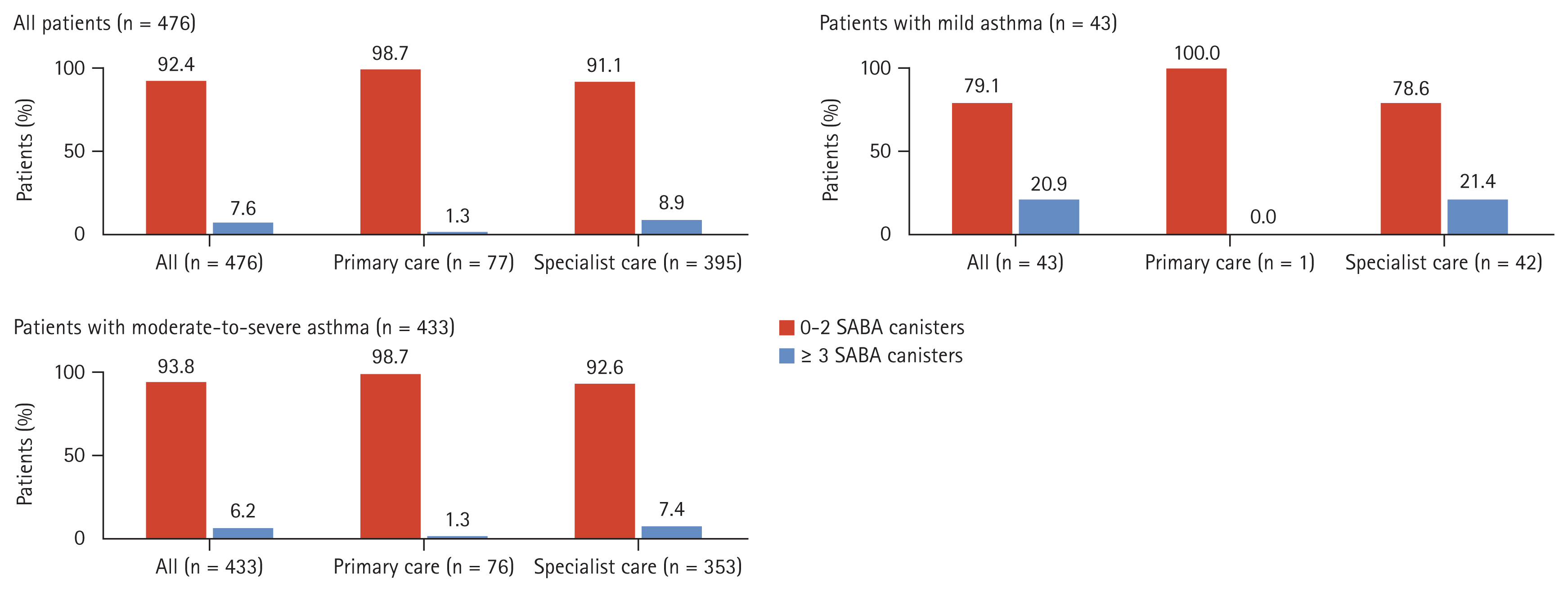
Table 1
| Vairable | All (n = 476) | All (n = 472)a) | |||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Primary care (n = 77) | Specialist care (n = 395) | ||||||
|
|
|
||||||
| Mild asthma (n = 1) | Moderate-to-severe asthma (n = 76) | All (n = 77) | Mild asthma (n = 42) | Moderate-to-severe asthma (n = 353) | All (n = 395) | ||
| Age (yr) | |||||||
|
|
|||||||
| Mean ± SD | 55.4 ± 15.1 | 81.0 ± NA | 52.3 ± 15.1 | 52.7 ± 15.4 | 51.9 ± 16.9 | 56.4 ± 14.8 | 55.9 ± 15.1 |
|
|
|||||||
| Age group (yr)b) | |||||||
|
|
|||||||
| 18–55 | 199 (41.8) | 0 (0.0) | 43 (56.6) | 43 (55.8) | 20 (47.6) | 135 (38.2) | 155 (39.2) |
|
|
|||||||
| ≥ 55 | 277 (58.2) | 1 (100.0) | 33 (43.4) | 34 (44.2) | 22 (52.4) | 218 (61.8) | 240 (60.8) |
|
|
|||||||
| Sex | |||||||
|
|
|||||||
| Female | 300 (63.0) | 1 (100.0) | 49 (64.5) | 50 (64.9) | 24 (57.1) | 223 (63.2) | 247 (62.5) |
|
|
|||||||
| BMI (kg/m2) | |||||||
|
|
|||||||
| Mean ± SD | 24.7 ± 4.0 | 27.7 ± NA | 24.0 ± 3.6 | 24.0 ± 3.6 | 24.6 ± 3.6 | 24.9 ± 4.1 | 24.9 ± 4.0 |
|
|
|||||||
| BMI group (kg/m2)c) | |||||||
|
|
|||||||
| < 18.5 | 18 (3.8) | 0 (0.0) | 4 (5.3) | 4 (5.2) | 1 (2.4) | 13 (3.7) | 14 (3.5) |
|
|
|||||||
| 18.5–22.9 | 150 (31.5) | 0 (0.0) | 29 (38.2) | 29 (37.7) | 14 (33.3) | 106 (30.0) | 120 (30.4) |
|
|
|||||||
| 23.0–24.9 | 105 (22.1) | 0 (0.0) | 19 (25.0) | 19 (24.7) | 8 (19) | 76 (21.5) | 84 (21.3) |
|
|
|||||||
| ≥ 25.0 | 203 (42.6) | 1 (100.0) | 24 (31.6) | 25 (32.5) | 19 (45.2) | 158 (44.8) | 177 (44.8) |
|
|
|||||||
| Education level | |||||||
|
|
|||||||
| Primary and/or secondary school | 52 (10.9) | 0 (0.0) | 5 (6.6) | 5 (6.5) | 4 (9.5) | 42 (11.9) | 46 (11.6) |
|
|
|||||||
| High school | 71 (14.9) | 0 (0.0) | 12 (15.8) | 12 (15.6) | 5 (11.9) | 54 (15.3) | 59 (14.9) |
|
|
|||||||
| University and/or post-graduate education | 138 (29.0) | 0 (0.0) | 35 (46.1) | 35 (45.5) | 17 (40.5) | 85 (24.1) | 102 (25.8) |
|
|
|||||||
| Unknown | 215 (45.2) | 1 (100.0) | 24 (31.6) | 25 (32.5) | 16 (38.1) | 172 (48.7) | 188 (47.6) |
|
|
|||||||
| Healthcare insurance/medication funding | |||||||
|
|
|||||||
| Not reimbursed | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
|
|
|||||||
| Partially reimbursed | 429 (90.1) | 1 (100.0) | 75 (98.7) | 76 (98.7) | 41 (97.6) | 308 (87.3) | 349 (88.4) |
|
|
|||||||
| Fully reimbursed | 5 (1.1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (1.4) | 5 (1.3) |
|
|
|||||||
| Unknown | 42 (8.8) | 0 (0.0) | 1 (1.3) | 1 (1.3) | 1 (2.4) | 40 (11.3) | 41 (10.4) |
|
|
|||||||
| Smoking status history | |||||||
|
|
|||||||
| Active smoker | 52 (10.9) | 0 (0.0) | 8 (10.5) | 8 (10.4) | 1 (2.4) | 43 (12.2) | 44 (11.1) |
|
|
|||||||
| Former smoker | 106 (22.3) | 0 (0.0) | 8 (10.5) | 8 (10.4) | 12 (28.6) | 86 (24.4) | 98 (24.8) |
| Never smoker | 318 (66.8) | 1 (100.0) | 60 (78.9) | 61 (79.2) | 29 (69.0) | 224 (63.5) | 253 (64.1) |
|
|
|||||||
| Asthma duration (yr) | |||||||
|
|
|||||||
| Mean ± SD | 6.5 ± 6.1 | 2.0 ± NA | 5.6 ± 4.5 | 5.6 ± 4.5 | 5.4 ± 4.2 | 6.9 ± 6.5 | 6.7 ± 6.3 |
|
|
|||||||
| Median (range) | 5.0 (1.0–52.0) | 2.0 (2.0–2.0) | 4.5 (1.0–22.0) | 4.0 (1.0–22.0) | 4.0 (1.0–17.0) | 5.0 (1.0–52.0) | 5.0 (1.0–52.0) |
|
|
|||||||
| GINA treatment stepd) | |||||||
|
|
|||||||
| Step 1 | 10 (2.1) | 1 (100.0) | 0 (0.0) | 1 (1.3) | 9 (21.4) | 0 (0.0) | 9 (2.3) |
|
|
|||||||
| Step 2 | 33 (6.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 33 (78.6) | 0 (0.0) | 33 (8.4) |
|
|
|||||||
| Step 3 | 202 (42.4) | 0 (0.0) | 40 (52.6) | 40 (51.9) | 0 (0.0) | 160 (45.3) | 160 (40.5) |
|
|
|||||||
| Step 4 | 181 (38.0) | 0 (0.0) | 33 (43.4) | 33 (42.9) | 0 (0.0) | 146 (41.4) | 146 (37.0) |
|
|
|||||||
| Step 5 | 50 (10.5) | 0 (0.0) | 3 (3.9) | 3 (3.9) | 0 (0.0) | 47 (13.3) | 47 (11.9) |
|
|
|||||||
| Comorbidities | |||||||
|
|
|||||||
| None | 73 (15.3) | 0 (0.0) | 17 (22.4) | 17 (22.1) | 7 (16.7) | 48 (13.6) | 55 (13.9) |
|
|
|||||||
| 1–2 | 231 (48.5) | 0 (0.0) | 38 (50.0) | 38 (49.4) | 22 (52.4) | 169 (47.9) | 191 (48.4) |
|
|
|||||||
| 3–4 | 110 (23.1) | 1 (100.0) | 17 (22.4) | 18 (23.4) | 9 (21.4) | 83 (23.5) | 92 (23.3) |
|
|
|||||||
| ≥ 5 | 62 (13.0) | 0 (0.0) | 4 (5.3) | 4 (5.2) | 4 (9.5) | 53 (15.0) | 57 (14.4) |
BMI, body mass index; GINA, Global Initiative for Asthma; NA, not available; SD, standard deviation.
c) BMI is categorized according to the Asia-Pacific classification as underweight (< 18.5 kg/m2), normal (18.5–22.9 kg/m2), overweight (23.0–24.9 kg/m2), and obese (≥ 25.0 kg/m2) [17].
d) Based on the 2017 GINA recommendations [16].
Table 2
| Variable | All (n = 476) | All (n = 472)a,b) | |||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Primary care (n = 77) | Specialist care (n = 395) | ||||||
|
|
|
||||||
| Mild asthma (n = 1) | Moderate-to-severe asthma (n = 76) | All (n = 77) | Mild asthma (n = 42) | Moderate-to-severe asthma (n = 353) | All (n = 395) | ||
| Patients prescribed SABA in addition to maintenance therapy | |||||||
|
|
|||||||
| Yes | 76 (16.0) | 0 (0.0) | 4 (5.3) | 4 (5.2) | 9 (21.4) | 63 (17.8) | 72 (18.2) |
|
|
|||||||
| SABA canisters or inhalers prescribed in the past 12 monthsc) | |||||||
|
|
|||||||
| Mean ± SD | 6.4 ± 24.0 | NA | 53.5 ± 104.3 | 53.5 ± 104.3 | 4.8 ± 1.5 | 3.6 ± 4.5 | 3.8 ± 4.3 |
|
|
|||||||
| Median (range) | 2.0 (1.0–210.0) | NA | 1.5 (1.0–210.0) | 1.5 (1.0–210.0) | 5.0 (3.0–7.0) | 2.0 (1.0–30.0) | 2.0 (1.0–30.0) |
|
|
|||||||
| SABA canisters or inhalers prescribed in the past 12 months by groups | |||||||
|
|
|||||||
| Total | 76 | 0 | 4 | 4 | 9 | 63 | 72 |
|
|
|||||||
| 1–2 | 40 (52.6) | NA | 3 (75.0) | 3 (75.0) | 0 (0.0) | 37 (58.7) | 37 (51.4) |
|
|
|||||||
| 3–5 | 17 (22.4) | NA | 0 (0.0) | 0 (0.0) | 7 (77.8) | 10 (15.9) | 17 (23.6) |
|
|
|||||||
| 6–9 | 15 (19.7) | NA | 0 (0.0) | 0 (0.0) | 2 (22.2) | 13 (20.6) | 15 (20.8) |
|
|
|||||||
| 10–12 | NA | NA | NA | NA | NA | NA | NA |
|
|
|||||||
| ≥ 13 | 4 (5.3) | NA | 1 (25.0) | 1 (25.0) | 0 (0.0) | 3 (4.8) | 3 (4.2) |
|
|
|||||||
| Patients prescribed ICS monotherapyd) | |||||||
|
|
|||||||
| Yes | 30 (6.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 20 (47.6) | 10 (2.8) | 30 (7.6) |
|
|
|||||||
| ICS canisters or inhalers prescribed in the past 12 monthsc) | |||||||
|
|
|||||||
| Mean ± SD | 15.7 ± 40.0 | NA | NA | NA | 20.0 ± 48.0 | 7.2 ± 12.7 | 15.7 ± 40.0 |
|
|
|||||||
| Median (range) | 4.5 (1.0–217.0) | NA | NA | NA | 6.0 (1.0–217.0) | 3.0 (1.0–42.0) | 4.5 (1.0–217.0) |
|
|
|||||||
| Total daily ICS dose prescribed | |||||||
|
|
|||||||
| Total | 30 | 0 | 0 | 0 | 20 | 10 | 30 |
|
|
|||||||
| Low dose | 24 (80.0) | NA | NA | NA | 18 (90.0) | 6 (60.0) | 24 (80.0) |
|
|
|||||||
| Medium dose | 6 (20.0) | NA | NA | NA | 2 (10.0) | 4 (40.0) | 6 (20.0) |
|
|
|||||||
| High dose | NA | NA | NA | NA | NA | NA | NA |
|
|
|||||||
| Patients prescribed ICS-LABA fixed-dose combination | |||||||
|
|
|||||||
| Yes | 454 (95.4) | 1 (100.0) | 75 (98.7) | 76 (98.7) | 22 (52.4) | 352 (99.7) | 374 (94.7) |
|
|
|||||||
| Total daily ICS dose prescribed | |||||||
|
|
|||||||
| Total | 454 | 1 | 75 | 76 | 22 | 352 | 374 |
|
|
|||||||
| Low dose | 226 (50.0) | 0 (0.0) | 53 (70.7) | 53 (69.7) | 12 (57.1) | 158 (45.0) | 170 (45.7) |
| Medium dose | 179 (39.6) | 1 (100.0) | 20 (26.7) | 21 (27.6) | 9 (42.9) | 148 (42.2) | 157 (42.2) |
|
|
|||||||
| High dose | 47 (10.4) | 0 (0.0) | 2 (2.7) | 2 (2.6) | 0 (0.0) | 45 (12.8) | 45 (12.1) |
|
|
|||||||
| Missing valuese) | 2 | 0 | 0 | 0 | 1 | 1 | 2 |
|
|
|||||||
| Patients prescribed OCS burst treatment/short coursef) | |||||||
|
|
|||||||
| Yes | 119 (25.0) | 0 (0.0) | 25 (32.9) | 25 (32.5) | 5 (11.9) | 87 (24.6) | 92 (23.3) |
|
|
|||||||
| Patients prescribed long-term OCS as maintenance treatmentg) | |||||||
|
|
|||||||
| Yes | 32 (6.7) | 0 (0.0) | 3 (3.9) | 3 (3.9) | 1 (2.4) | 28 (7.9) | 29 (7.3) |
|
|
|||||||
| Patients prescribed antibiotics for asthma | |||||||
|
|
|||||||
| Yes | 63 (13.3) | 0 (0.0) | 20 (26.3) | 20 (26.0) | 3 (7.1) | 40 (11.4) | 43 (10.9) |
|
|
|||||||
| Missing valuese) | 2 | 0 | 0 | 0 | 0 | 2 | 2 |
ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; NA, not available; OCS, oral corticosteroid; SABA, short-acting β2-agonist; SD, standard deviation.
b) Only 1 patient with mild asthma, who was treated in specialist care, was prescribed SABA monotherapy, with a prescription of 1–2 SABA canisters in the past 12 months.
Table 3
| Variable | All (n = 476) | All (n = 472)b) | |||||
|---|---|---|---|---|---|---|---|
|
|
|||||||
| Primary care (n = 77) | Specialist care (n = 395) | ||||||
|
|
|
||||||
| Mild asthma (n = 1) | Moderate-to-severe asthma (n = 76) | All (n = 77) | Mild asthma (n = 42) | Moderate-to-severe asthma (n = 353) | All (n = 395) | ||
| Number of severe asthma exacerbations in the past 12 months | |||||||
|
|
|||||||
| Mean ± SD | 0.5 ± 1.1 | 0.0 ± NA | 0.8 ± 1.5 | 0.8 ± 1.4 | 0.2 ± 0.7 | 0.5 ± 1.1 | 0.5 ± 1.0 |
|
|
|||||||
| Median (range) | 0.0 (0.0–8.0) | 0.0 (0.0–0.0) | 0.0 (0.0–8.0) | 0.0 (0.0–8.0) | 0.0 (0.0–4.0) | 0.0 (0.0–8.0) | 0.0 (0.0–8.0) |
|
|
|||||||
| Number of severe asthma exacerbations in the past 12 months by groups | |||||||
|
|
|||||||
| None | 335 (70.4) | 1 (100.0) | 49 (64.5) | 50 (64.9) | 36 (85.7) | 247 (70.0) | 283 (71.6) |
|
|
|||||||
| 1 | 86 (18.1) | 0 (0.0) | 12 (15.8) | 12 (15.6) | 4 (9.5) | 69 (19.5) | 73 (18.5) |
|
|
|||||||
| 2 | 30 (6.3) | 0 (0.0) | 7 (9.2) | 7 (9.1) | 1 (2.4) | 22 (6.2) | 23 (5.8) |
|
|
|||||||
| 3 | 10 (2.1) | 0 (0.0) | 4 (5.3) | 4 (5.2) | 0 (0.0) | 6 (1.7) | 6 (1.5) |
|
|
|||||||
| > 3 | 15 (3.2) | 0 (0.0) | 4 (5.3) | 4 (5.2) | 1 (2.4) | 9 (2.5) | 10 (2.5) |
|
|
|||||||
| Level of asthma symptom control | |||||||
|
|
|||||||
| Well-controlled | 294 (61.8) | 1 (100.0) | 26 (34.2) | 27 (35.1) | 35 (83.3) | 230 (65.2) | 265 (67.1) |
|
|
|||||||
| Partly controlled | 142 (29.8) | 0 (0.0) | 45 (59.2) | 45 (58.4) | 5 (11.9) | 91 (25.8) | 96 (24.3) |
|
|
|||||||
| Uncontrolled | 40 (8.4) | 0 (0.0) | 5 (6.6) | 5 (6.5) | 2 (4.8) | 32 (9.1) | 34 (8.6) |
a) Severe asthma exacerbations in the 12 months before the study visit were defined based on the European Respiratory Society/American Thoracic Society guidelines as a worsening of asthma symptoms requiring hospitalization, an emergency room visit, or the need for intravenous corticosteroids or OCS for ≥ 3 days or a single intramuscular corticosteroid dose.
REFERENCES
- TOOLS



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement figure 1
Supplement figure 1 Print
Print



