 |
 |
| Korean J Intern Med > Epub ahead of print |
|
Abstract
Notes
Figure┬Ā1
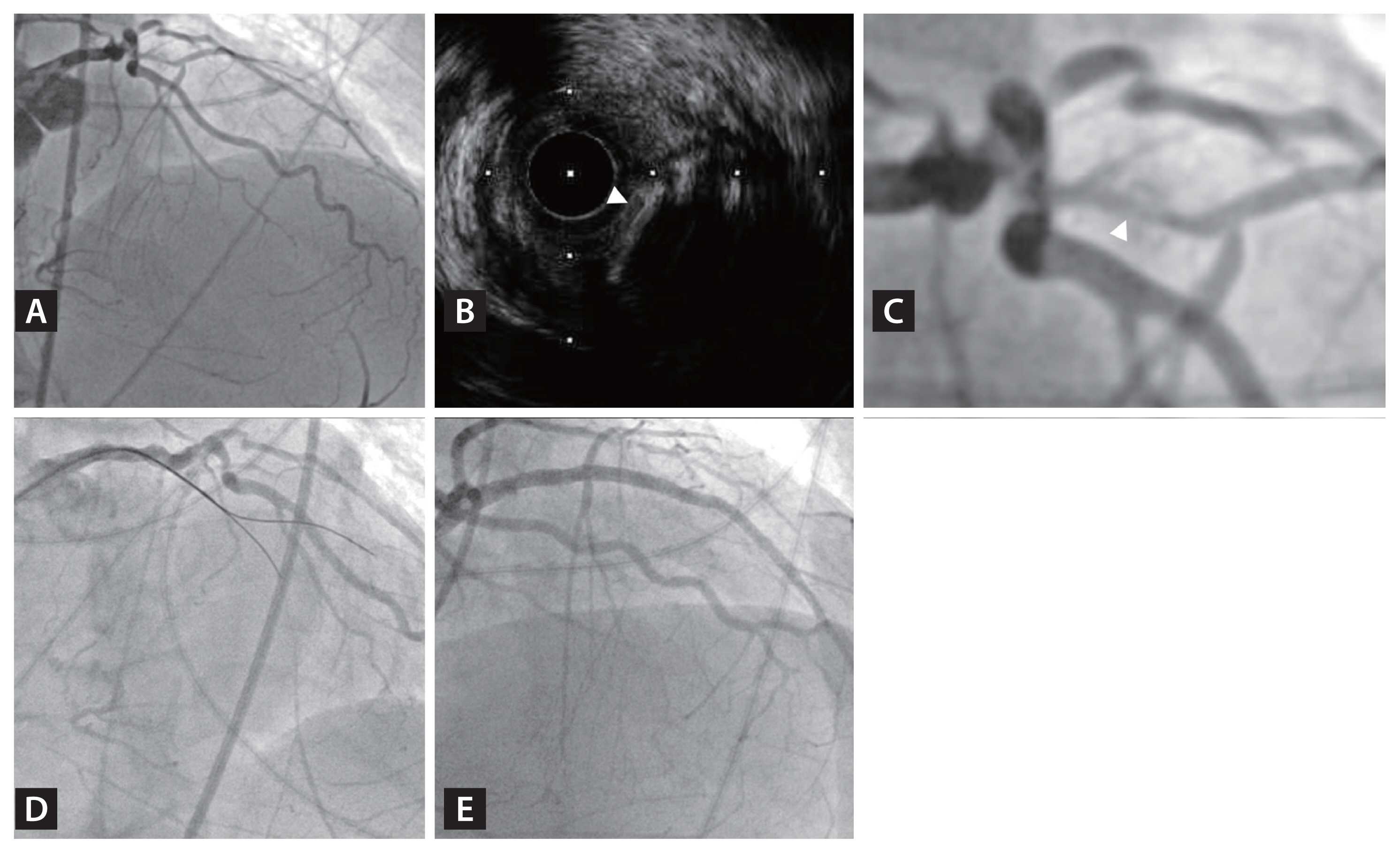
Figure┬Ā2

Figure┬Ā3
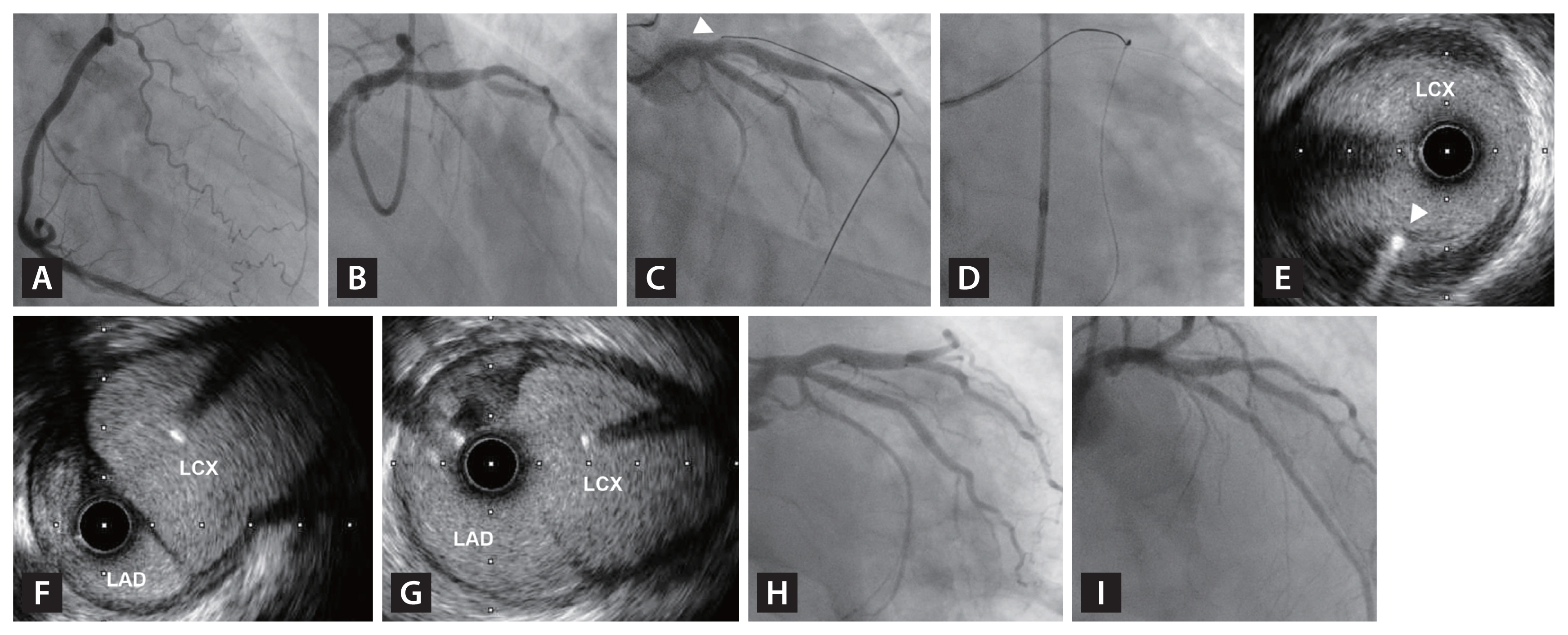
Figure┬Ā4
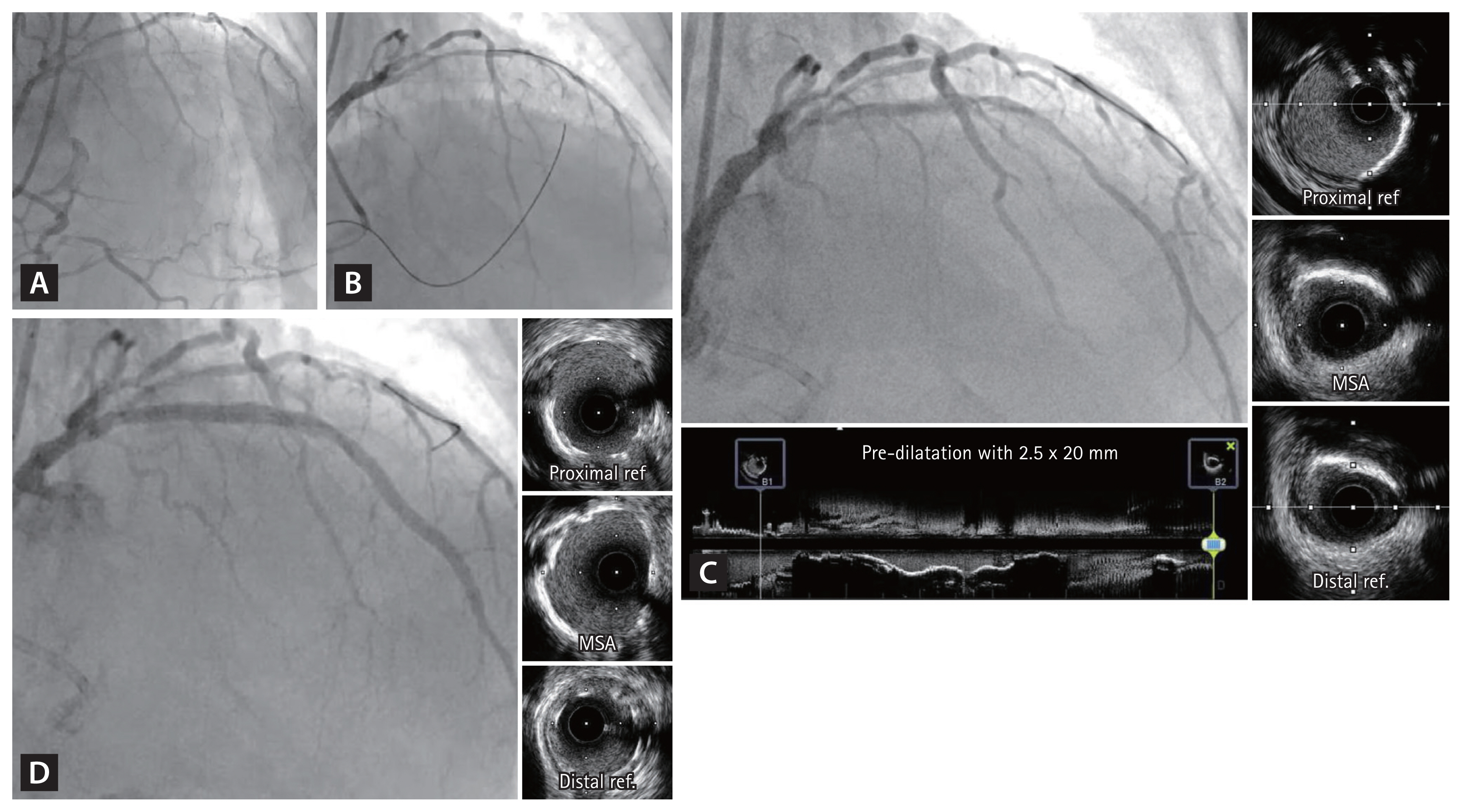
Figure┬Ā5
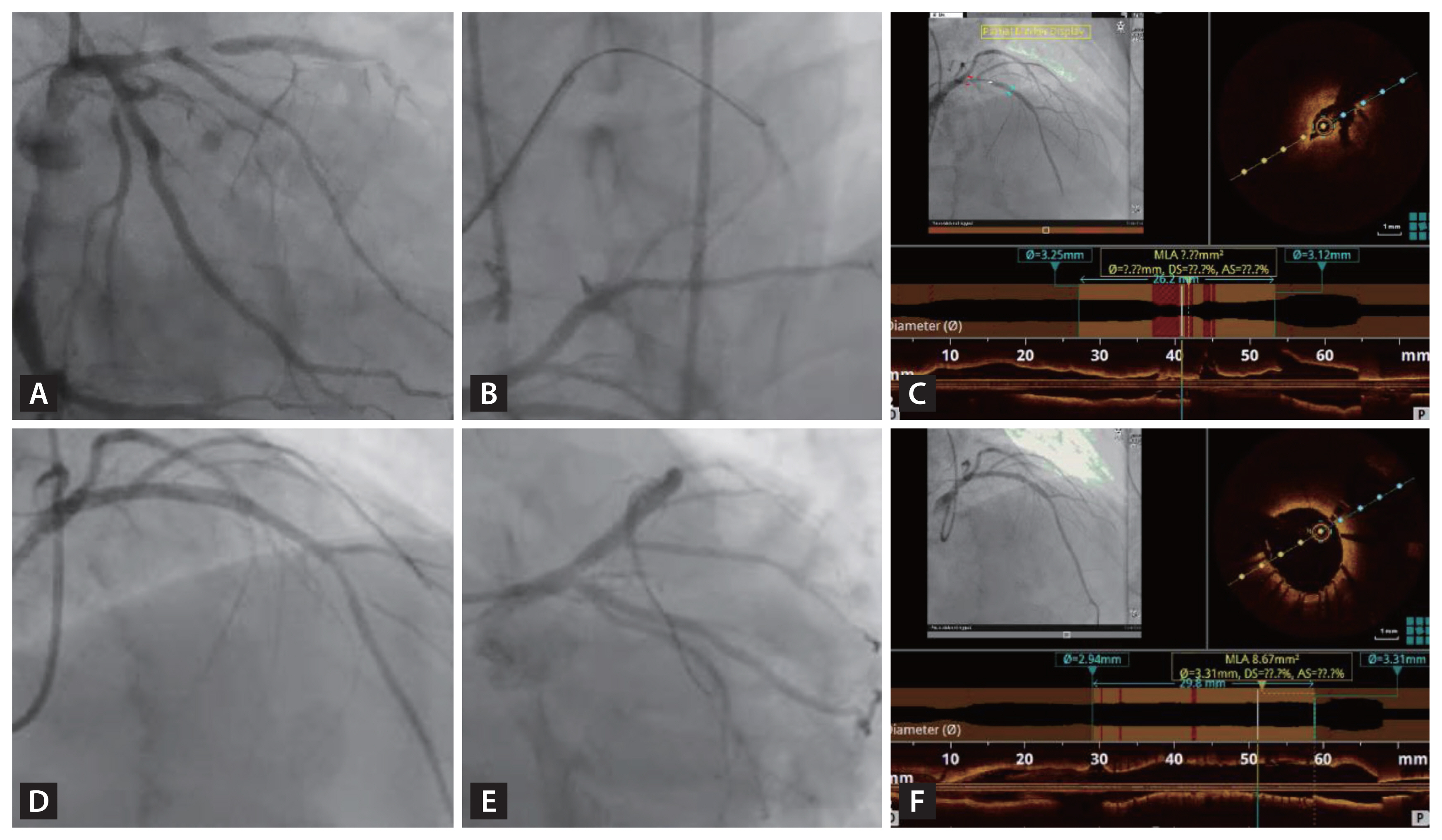
Figure┬Ā6
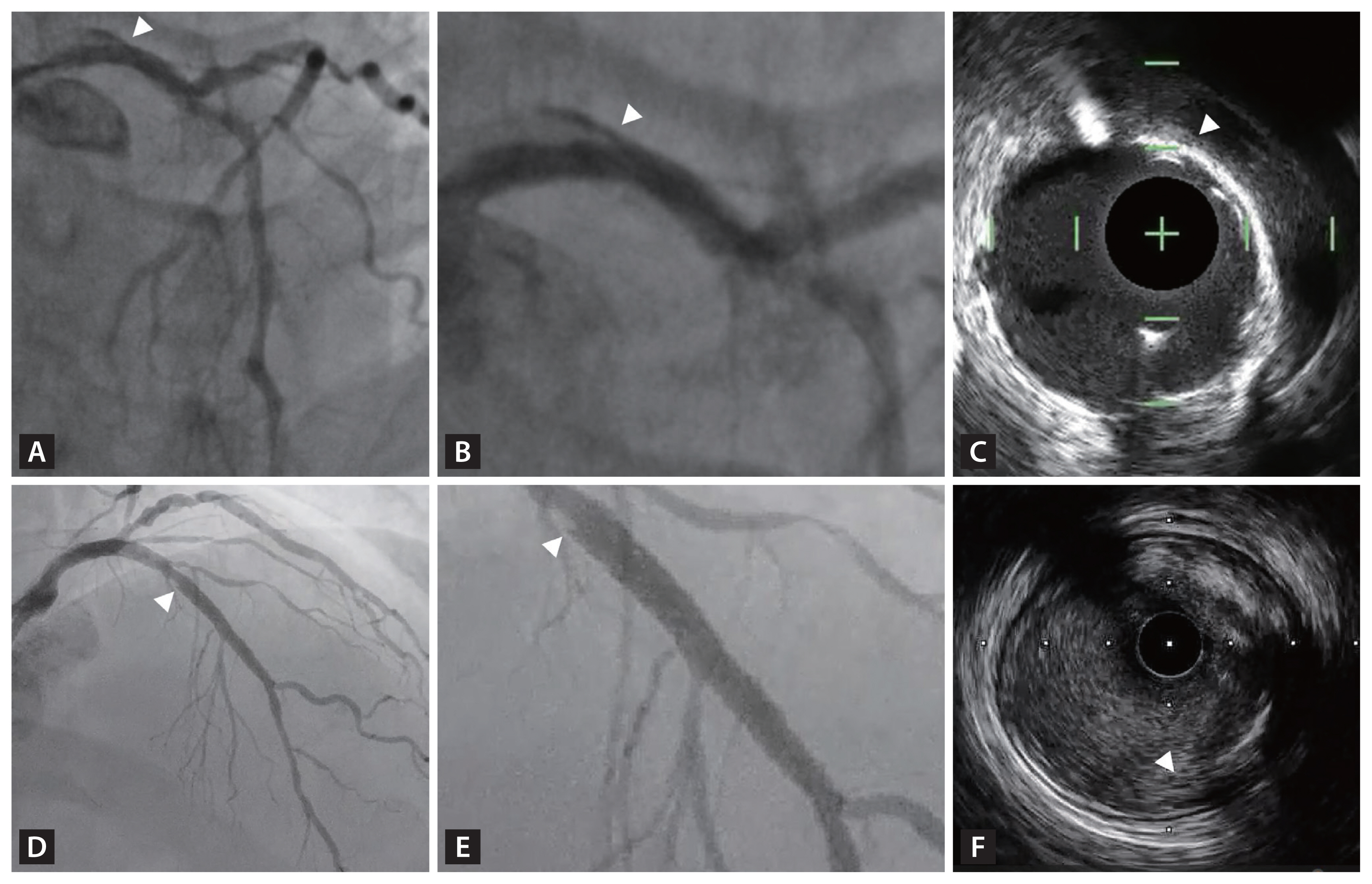
Figure┬Ā7
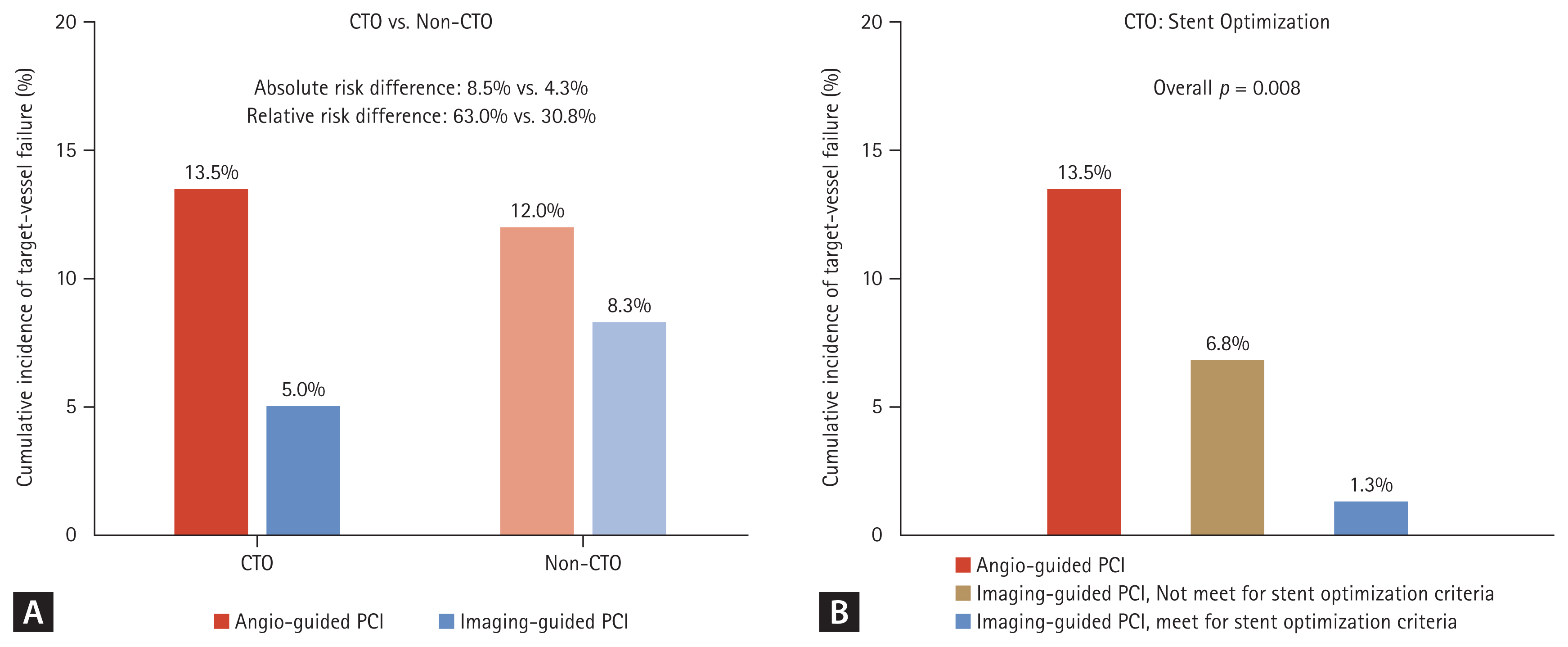
Figure┬Ā8
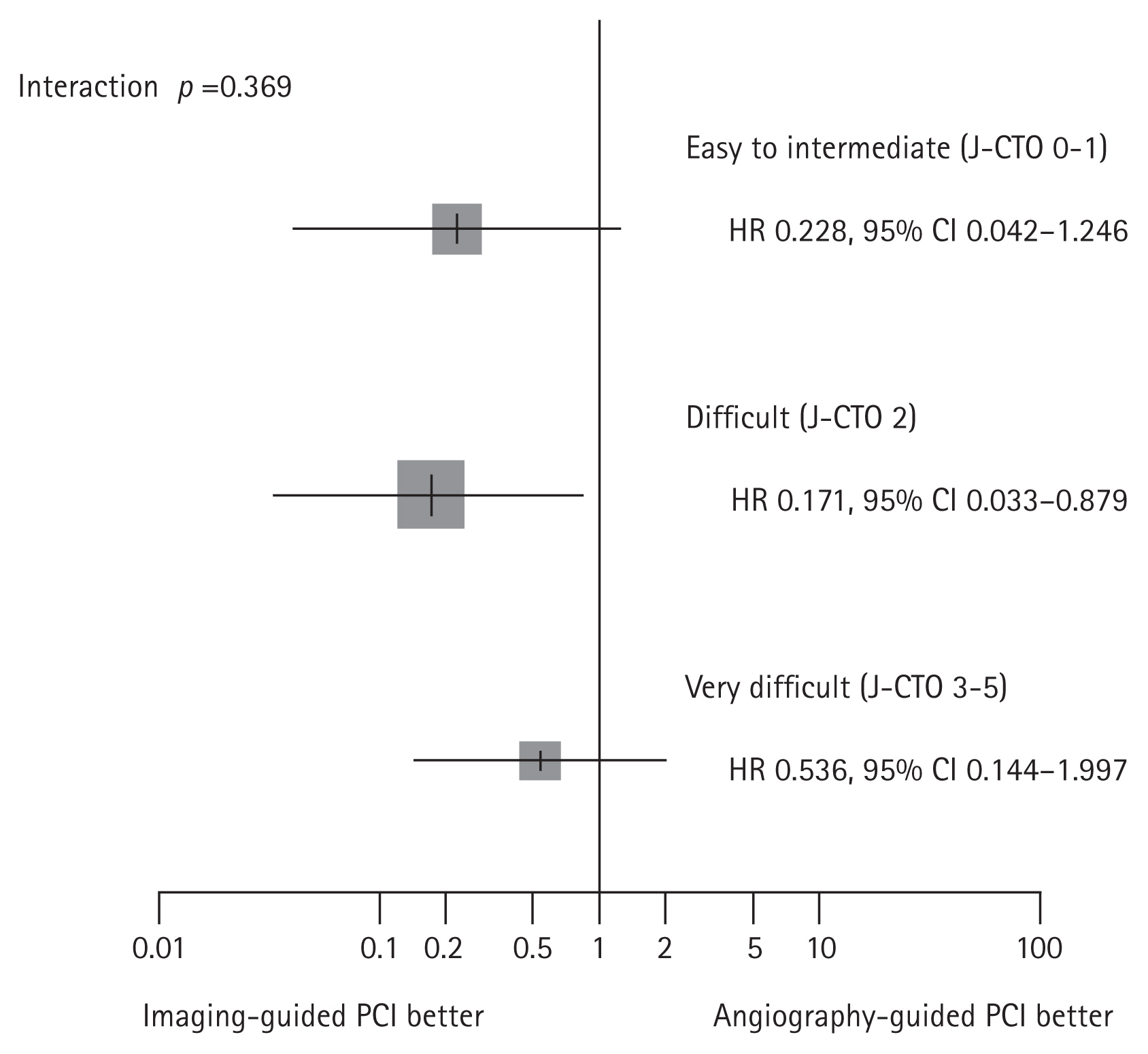
Figure┬Ā9

Table┬Ā1
| Study | Imaging modality | Patient number | Design | Main findings |
|---|---|---|---|---|
| Dai et al. [19] (2013) | IVUS | 49 | Retrospective | Single center data (2006ŌĆō2012). In patients with previous failed attempts by anterograde and/or retrograde wiring, IVUS-guided reverse CART provided high success rates (95.9%). Complication rate was 10.2% without clinically relevant adverse events. In-hospital MACE occurred in only 1 case (2.0%) with non-ST segment elevation MI. |
| Christakopoulos et al. [20] (2016) | NIRS | 15 | Retrospective | In patients with successful wire cross in CTO, NIRS was performed. Large LCP was defined as max LCBI in a 4 mm length of artery (maxLCBI 4 mm) Ōēź 500. Large LCP seen in 4 of 15 CTO vessels (27%). The 12-month incidence of in-stent restenosis and target-lesion revascularization was 25%, and all patients who developed restenosis had LCP at baseline. |
| Karacsonyi et al. [21] (2016) | IVUS or OCT | 619 | Retrospective | Among 7 US centers (2012ŌĆō2015), IVUS (36%) and OCT (3%) were used in CTO PCI. Intravascular imaging was more frequently used in lesions with proximal cap ambiguity and retrograde crossing. Intravascular imaging was used for lesion crossing (35.7%) and stent sizing/optimization (64.3%). Success rates were similar with or without intravascular imaging (92.8% vs. 89.6%, p = 0.302) despite higher complexity in cases with intravascular imaging (J-CTO score: 2.86 vs. 2.43). Inhospital MACE rates were comparable with or without intravascular imaging (2.7% vs. 3.2%, p = 0.772). |
| Song et al. [16] (2017) | IVUS | 219 | Retrospective | Single center data (2014ŌĆō2016). In patients with successful recanalization of CTO, actual cross mechanism of CTO segment (subintima tracking vs. true lumen wiring) was re-evaluated using IVUS. Subintimal tracking was detected in 52.1% (86.7% in dissection re-entry and 27.9% in wire escalation cases). IVUS-defined subintimal tracking group had higher rates of in-hospital MACE (7.9% vs. 1.9%, p = 0.04), greater IVUS-detected vascular injury, angiographic dye staining/extravasation, and branch occlusion than IVUS-defined intraplaque tracking. |
| Finn et al. [22] (2019) | IVUS | 157 | Retrospective | Part of the same cohort with Song et al. [16] Single center data (2014ŌĆō2016) evaluating IVUS-defined subintimal vs. intraplaque tracking in patients with successful recanalization of CTO. One year follow-up results showed that unadjusted rate of TVF in the subintimal tracking group was higher than the intraplaque group (17.9 vs. 6.9%, HR 2.74, 95% CI 1.00ŌĆō7.54, p = 0.04), driven by numerically higher rates of TVR and peri-procedural MI. Multivariable analysis did not show any difference in TVF between the 2 groups. |
| Xhepa et al. [23] (2019) | OCT | 75 | Prospective | Among 75 patients with successful CTO recanalization, intraplaque vs. DART were used 46 and 29 patients, respectively. Follow-up angiography and OCT at 6ŌĆō9 months from index procedure revealed no significant difference in diameter stenosis and late lumen loss between intraplaque vs. DART. Although DART showed higher strut malapposition (13.6% vs. 6.6%, p < 0.001), but there was no significant difference in strut coverage (71.3% vs. 79.9%, p = 0.255) than intraplaque tracking. |
| Kalogeropoulos et al. [24] (2021) | IVUS | 514 | Retrospective | Single center data (2012ŌĆō2018). Among 514 patients with successful CTO PCI, IVUS-guided PCI was performed in 184 (35.8%) of cases. Propensity score matching analysis compared 182 patients in each IVUS-guided vs. angiography-guided CTO PCI group. IVUS-guided PCI group had larger stent diameter (3.5 mm vs. 3.2 mm, p < 0.001), longer total stent length (60.0 mm vs. 38.0 mm, p < 0.001), and procedure time. There was no difference in the incidence of the composite endpoint of all-cause death, cardiac death, MI and TVR between the IVUS-guided PCI and the angiography-guided PCI groups (13.7% vs. 15.9%, log-rank p = 0.67) during median follow-up of 49 months. |
| Maknojia et al. [25] (2023) | IVUS or OCT | 33,345 | Retrospective | Nationwide Inpatient Sample database analysis from 2015 to 2018. Among 33,345 patients who underwent single vessel CTO PCI, 8.8% of patients used IVUS/OCT. In-hospital mortality was similar between patients with and without intravascular imaging in CTO PCI (1.5% vs. 1.3%, p = 0.195). The cost of hospitalization was higher in intravascular imaging group in CTO PCI (IVUS/OCT vs. no IVUS/OCT: $27,427 vs. $21,452, p < 0.001; IVUS vs. no IVUS: $27,172 vs. $21,478, p < 0.001; OCT vs. no OCT: $34,166 vs. $21,775, p < 0.001). |
| Karacsonyi et al. [26] (2023) | IVUS | 8,771 | Retrospective | 8,771 patients enrolled in the PROGRESS-CTO Registry (NCT02061436) from 2012ŌĆō2022 at 39 centers. IVUS was used in 44.5% of cases (11.5% for crossing, 33.1% for stent optimization, and 6.0% for both crossing and stent optimalization). IVUS was used more frequently in cases with calcification, in-stent restenosis CTO, higher J-CTO and PROGRESS-CTO scores. IVUS use resulted in about 35 minutes longer procedural time, but lower radiation dose and contrast volume, and higher technical (99% vs. 96%, p < 0.001) as well as procedural success rates (96% vs. 95%, p = 0.002). In-hospital MACE was comparable (2.04% vs. 1.62%, p = 0.176). |
CART, controlled antegrade and retrograde tracking; CI, confidence interval; CTO, chronic total occlusion; DART, dissection and reentry techniques; HR, hazard ratio; IVUS, intravascular ultrasound; J-CTO, Japanese-CTO; LCBI, lipid-core burden index; LCP, lipid-core plaque; MACE, major adverse cardiovascular events; MI, myocardial infarction; NIRS, near-infrared spectroscopy; OCT, optical coherence tomography; PCI, percutaneous coronary intervention; TVF, target-vessel failure; TVR, target-vessel revascularization.
Table┬Ā2
| Study | Imaging modality | Patient number | Primary end point | Clinical end point | Main findings | Limitations |
|---|---|---|---|---|---|---|
| AIR-CTO [8] | IVUS | 230 | In-stent LLL at 1-year | MACE (all-cause death, MI, repeat revascularization by PCI or CABG, stent thrombosis) at 2-years |
IVUS-guided PCI significantly reduced in-stent LLL than angiography-guided PCI (0.28 ┬▒ 0.48 mm vs. 0.46 ┬▒ 0.68 mm, p = 0.025). MACE risk was similar between the 2 groups (21.7% vs. 25.2%, p = 0.641). |
Not powered for clinical outcome |
| CTO-IVUS [9] | IVUS | 402 | Cardiac death at 1-year | MACE (cardiac death, MI, TVR) at 1-year |
Cardiac death was not significantly different between IVUS-guided PCI and angiography-guided PCI (0% vs. 1.0%, p = 0.16). MACE was significantly lower in IVUS-guided PCI than angiography-guided PCI (2.6% vs. 7.1%; HR 0.35; 95% CI 0.13ŌĆō0.97; p = 0.035). |
2 ├Ś 2 design of 1st arm (IVUS vs. no-IVUS) and 2nd arm (Endeavor Resolute vs. Nobori). Failed to prove primary end point. |
| Substudy of the RENOVATE-COMPLEXPCI [7,10] | IVUS or OCT | 319 | Target vessel failure (cardiac death, TV-MI, and TVR) at median 2.1 years | Target vessel failure (cardiac death, TV-MI, and TVR) at median 2.1 years | The risk of TVF was significantly lower in the intravascular imaging-guided PCI than angiography-guided PCI (5.0% vs. 13.5%; HR 0.30; 95% CI 0.13ŌĆō0.71; p = 0.006). | Prespecified substudy of main trial |
CABG, coronary artery bypass grafting; CI, confidence interval; CTO, chronic total occlusion; HR, hazard ratio; IVUS, intravascular ultrasound; LLL, late lumen loss; MACE, major adverse cardiovascular events; MI, myocardial infarction; OCT, optical coherence tomography; PCI, percutaneous coronary intervention; TVR, target vessel revascularization.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



