 |
 |
| Korean J Intern Med > Volume 39(3); 2024 > Article |
|
Abstract
Background/Aims
The reimbursement policy for cryptogenic stroke (CS) was expanded in November 2018 from recurrent strokes to the first stroke episode. No reports have demonstrated whether this policy change has affected trends in implantable loop recorder (ILR) utilization.
Methods
We identified patients who received an ILR implant using the Korea Health Insurance Review and Assessment Service database between July 2016 and October 2021. Patients meeting all the following criteria were considered to have CS indication: 1) prior stroke history, 2) no previous history of atrial fibrillation or flutter (AF/AFL), and 3) no maintenance of oral anticoagulant for ≥4 weeks within a year before ILR implant. AF/AFL diagnosed within 3 years after ILR implant or before ILR removal was considered ILR-driven.
Results
Among 3,056 patients, 1,001 (32.8%) had CS indications. The total ILR implant number gradually increased for both CS and non-CS indications and the number of CS indication significantly increased after implementing the expanded reimbursement policy. The detection rate for AF/AFL was 26.3% in CS patients over 3 years, which was significantly higher in patients implanted with an ILR within 2 months after stroke than those implanted later.
Conclusions
The expanded coverage policy for CS had a significant impact on the number of ILR implantation for CS indication. The diagnostic yield of ILR for AF/AFL detection seems better when ILR is implanted within 2 months than later. Further investigation is needed to demonstrate other clinical benefits and the optimal ILR implantation timing.
Implantable loop recorders (ILRs) are small cardiac devices inserted into subcutaneous tissue to detect and record arrhythmic events for up to 3 years. The most common indications for ILR implantation are unexplained syncope, palpitation, and cryptogenic stroke (CS). In two real-world registries, ILRs were most frequently implanted because of syncope [1,2], but ILR also has proven its efficacy to detect atrial fibrillation in CS patients [3,4].
ILR implantation is currently reimbursed for 3 different indications in Korea: recurrent syncope, palpitation of unexplained cause, and CS. On November 2018, the reimbursement coverage for CS was expanded from “recurrent” to the first stroke episode. However, no nationwide reports have demonstrated whether this policy change has affected ILR utilization trends. We analyzed time-series trends in ILR implant before and after reimbursement policy change using the Korean Health Insurance Review & Assessment Service (HIRA) database. We also evaluated the diagnostic yield of ILR to detect atrial fibrillation or flutter (AF/AFL) in CS patients.
We obtained data from patients who were implanted with ILR between July 2016 and October 2021 from the HIRA database. HIRA collects medical information including patient’ age and sex, medical or surgical treatment, and diagnoses, which are coded according to the International Classification of Disease, Tenth Revision (ICD-10). The database, which includes almost the entire Korean population, is available for researchers through the healthcare big data open system [5].
ILR implant was identified with the corresponding National Health Insurance (NHI) procedure codes (E6551 and E6553). ILR implant indications were classified to either CS or non-CS. CS was defined if all the following criteria were met: 1) stroke prior to ILR implant, 2) no history of AF/AFL before ILR implant, and 3) no maintenance of oral anticoagulant for ≥ 4 weeks within a year before ILR implant. The first diagnosis date of stroke (ICD-10: I63 or I64) was considered as the date of index stroke. Patients with CS indication were classified into 2 groups according to the interval from the index stroke to ILR implant; early implant (≤ 2 mo) and late implant (> 2 mo).
Baseline characteristics were identified using the ICD-10 and prescription codes. A history of hypertension, diabetes mellitus, stroke, heart failure, cardiomyopathy, AF/AFL, myocardial infraction, peripheral arterial disease, and chronic kidney disease were assessed. We also assessed diagnostic tests before ILR implant using the NHI procedure code, which include 12-lead electrocardiogram, Holter-monitoring, transcranial Doppler, and trans-thoracic or -esophageal echocardiography. The primary outcome was temporal trends in ILR utilization following the expanded coverage policy for ILR implantation according to implant indication (CS or non-CS). We also investigated AF/AFL incidence to assess the diagnostic yield of ILR in CS patients as a secondary outcome. Atrial fibrillation or flutter diagnosed within 3 years after ILR implant or before ILR removal was considered ILR-driven.
Categorical variables were presented as numbers and percentages and continuous variables were shown as means with standard deviation. We used the χ2 test for comparison of categorical variables and the t-test for continuous variables. A segmented linear regression analysis of interrupted time-series was performed to estimate the impact of the policy change on ILR utilization for each non-CS and CS indication. Cumulative incidence was estimated using the Kaplan-Meier estimator for AF/AFL (and hazard ratios were determined using the log-rank test). p values less than 0.05 at a 2-sided significance level were considered significant. SAS 9.4 version (SAS institute Inc., Cary, NC, USA) was used for all statistical analyses.
A total of 3,056 individuals were implanted with ILR between July 2016 and October 2021. Among 1,404 patients with prior stroke, 1,001 (32.8%) were classified to have CS indication (Fig. 1). Baseline patient characteristics are shown in Table 1. The mean age of the total population was 63 years and the prevalence of hypertension and diabetes were 76.9% and 63.8%, respectively.
Compared to those with non-CS indication, patients with CS were older, more frequently men, and had higher prevalence of comorbidities including hypertension, diabetes, peripheral artery disease, and chronic kidney disease. The CHA2DS2VASc score was also significantly higher in patients with CS than in those without (4.3 ± 1.7 vs. 3.3 ± 2.0, p < 0.001). Before ILR implant, the majority of patients underwent rhythm monitoring using 12-lead electrocardiogram or Holter monitoring, while echocardiography was performed in only about half the patients. Holter monitoring and trans-esophageal echocardiography were performed more frequently in patients with CS than in those with non-CS indication.
The total number of ILR implants for each indication has gradually increased over time except for a period of stagnation during the COVID-19 pandemic and a noticeable jump after the policy change. The ratio of CS indications to total indications has also gradually increased following the introduction of the new expanded policy, while that of non-CS has remained stationary (Fig. 2). In the CS indication group, the implant number significantly increased by 6.2 per 4 months (p = 0.025), and the slope of the implant number increased but without statistical significance (p = 0.073) after the policy change. The implant number in the non-CS indication group increased significantly before the policy change (p < 0.001), but it became insignificant after the policy change (Table 2, Fig. 3).
Among 1,001 patients with CS indication, 844 (84.3%) underwent ILR implantation after the policy change, and 232 (23.2%) underwent implantation within 2 months after their index stroke. Patients implanted with ILR after the policy change were younger and showed lower prevalences of diabetes and heart failure resulting in lower CHA2DS2VASc score compared with those implanted before the policy change. The mean time between stroke and ILR was 3.6 years, which was significantly longer before than after the policy change (4.5 vs. 3.5 yr, p = 0.007). The rate of early implant (≤ 2 months after index stroke) was also significantly higher in those who underwent ILR implant after the policy change (25.2% vs. 12.1%, p < 0.001). Holter monitoring and trans-thoracic or -esophageal echocardiography were performed more frequently before than after the policy change (Table 3). Baseline patient characteristics according to the interval from the index stroke to ILR implant are shown in Supplementary Table 1.
During the first 3 months after ILR implant, AF/AFL was detected in 130 (13.0%) patients. The diagnosis rate at 3 years was 26.3% in all CS patients. Kaplan-Meier curves comparing the cumulative incidence of AF/AFL between the 2 groups according to implant time and interval from index stroke over a 3-year period are shown in Figure 4. There was no difference in AF/AFL incidence between patients who underwent ILR implantation before and after the policy change. However, the early implant group showed a significantly higher AF/AFL incidence compared with the late implant group (hazard ratio 1.47, 95% confidence interval 0.50–0.85, p = 0.001). The mean time intervals between stroke and anticoagulation initiation (13.6 vs. 72.1 mo, p < 0.001) and between ILR implant and anticoagulation initiation (12.7 vs. 13.4 mo, p = 0.048) were significantly shorter in the early implant compared to the late implant group.
This nationwide cohort study investigated the ILR utilization in Korea between July 2016 and October 2021. The main findings were: 1) total ILR implant number gradually increased for both CS and non-CS indications; 2) about a third of patients underwent ILR implantation for CS indication and the number of ILR implantation for CS indication significantly increased after the expanded reimbursement policy was implemented in November 2018; and 3) the diagnostic yield of ILR for AF/AFL detection was 26.3% in CS patients over 3 years, which was significantly higher among early implants compared to the late implant group.
In this study, we first evaluated whether implementation of a policy to allow ILR implant for the first stroke instead of only after recurrent stroke resulted in an increase in ILR usage in CS patients in Korea. The number of ILR implants for CS indication significantly increased after the policy change, and CS recently accounted for almost a third of all ILR implantation indications, which is a three-fold increase from when reimbursement began. The stroke-to-ILR implant interval also significantly decreased after the policy change, which possibly resulted in younger patients with ILR implants after the policy change than before.
There is a clinical significance of AF/AFL detection in CS patients because it has an impact on secondary stroke prevention, which is possibly associated with higher anticoagulation incidence, although controversial [6,7]. The AF/AFL detection rate in our cohort was similar or relatively higher than in previous studies, which showed that most AF detections by ILR occurred during the first few months [8–12]; however, the cumulative incidence of AF detection continued to increase over a year, albeit slowly. Our study results were also consistent with these findings, but the most distinct observation from our study population was that the interval from the index stroke to ILR implant was remarkably long [3,4,9–13]. This is not only because of the different study design and population in our study, but also because ILR implants were reimbursed for only recurrent stroke until November 2018 in Korea. The reason why the interval was substantially long even after the policy change is likely because “recurrent” stroke still accounted for a large portion of CS indications, especially in the early period after the policy change. This might explain why the AF/AFL detection rate was not different despite the fact that the expanded policy led to a significant increase in ILR implant for CS. Considering that the detection rate was significantly higher in the early than in the late implant group, it is possible that the policy change impacted the AF/AFL detection rate in CS patients. However, ILR still seems useful to detect AF/AFL in patients after a long interval between stroke and ILR implant, presumably those who experienced recurrent stroke, even though the diagnostic yield in AF detection was higher in patients who were implanted with ILR within 2 months after their stroke. The interval from the stroke to ILR implant was still long in patients with non-recurrent events (mean 1,289 d). We attribute this to the relatively late timing of ILR insertion for CS in Korea, partly because of possible time lag between policy change and implementation in clinical practice.
A significant portion of CSs is attributed to cardioembolism. Cardioembolic stroke has a worse prognosis than strokes of other etiologies, and has higher risk of recurrence, severe disability, and mortality; thus, CS warrants comprehensive evaluation, particularly cardiac diagnostics [14–18]. However, echocardiography was performed in just over half of CS patients against our expectations, which was one of the major differences in our study from previous studies. Nevertheless, those studies included a relatively small number of patients from a single or limited number of centers or they had a prospective study design following a recommended protocol, while our study is a nationwide population-based retrospective cohort study [4,9,12,13,19]. Therefore, our study likely reflects the real-world practice for CS work-up in Korea. There is another possibility that other imaging modalities such as cardiac magnetic resonance imaging or computed tomography were performed as an alternative to echocardiography. For example, transcranial Doppler exam was performed in 29.6% of CS patients.
This study has several limitations. First, stroke etiology could not be confirmed because of the inherent limitations of claims data. The etiology of the study population is most likely to be cryptogenic considering the indications for ILR implantation coverage in Korea. We also incorporated 2 other criteria, including no prior history of AF/AFL and anticoagulation, to increase validity. However, patients with prior stroke, whether cryptogenic or not, could have a non-CS indication for ILR implantation. Second, we presumed that atrial fibrillation or flutter diagnosed after ILR implant was ILR-driven. However, we cannot confirm that all those events were detected by ILR nor which criteria were used for clinical diagnosis of AF/AFL in terms of duration [4]. Third, there was a chance of missing diagnoses of AF/AFL in patients without regular ILR interrogation. Lastly, we could not evaluate whether AF/AFL detection by ILR is beneficial for patient improving clinical outcomes including secondary stroke prevention.
This study investigated temporal trends and changes in ILR utilization following the expanded coverage policy for ILR implantation. We identified that the number of ILR implantations for CS indication significantly increased after the expanded reimbursement policy and CS accounted for a third of all indications. We also showed that the incidence of AF/AFL detection was higher when ILR was implanted within 2 months from the index stroke than after more than 2 months. We hope that our findings can be the basis for further studies to demonstrate the clinical benefit of AF/AFL detection using ILR and to determine the best timing of ILR implantation.
1. The total ILR implant number has gradually increased for both CS and non-CS indications.
2. The number of ILR implantation for CS indication significantly increased after the expanded reimbursement.
3. The diagnostic yield of ILR for atrial fibrillation detection was 26.3%, which was significantly higher when ILR was implanted within 2 months than later.
Acknowledgments
The authors appreciate the Korean Health Insurance Review & Assessment Service for providing the data.
Notes
CRedit authorship contributions
Hye Bin Gwag: conceptualization, methodology, investigation, formal analysis, validation, writing - original draft, writing - review & editing, project administration, funding acquisition; Nak Gyeong Ko: methodology, investigation, data curation, formal analysis; Mihyeon Jin: investigation, data curation, formal analysis
Figure 2
The number of ILR implantations for each indication. (A) Total number of ILR implants for each indication. (B) The ratio of CS and non-CS indications to total implantations. The dashed line indicates the implementation of the new expanded policy. ILR, implantable loop recorder; CS, cryptogenic stroke.

Figure 3
Trends in total ILR implant number every 4 months according to each indication. The vertical dashed line indicates the implementation of the new expanded policy. ILR, implantable loop recorder.

Figure 4
Cumulative incidences of AF/AFL detection according to (A) implant period and (B) timing in CS patients. AF/AFL, atrial fibrillation or flutter; CS, cryptogenic stroke. p value refers to the result of the log-rank test between 2 groups.
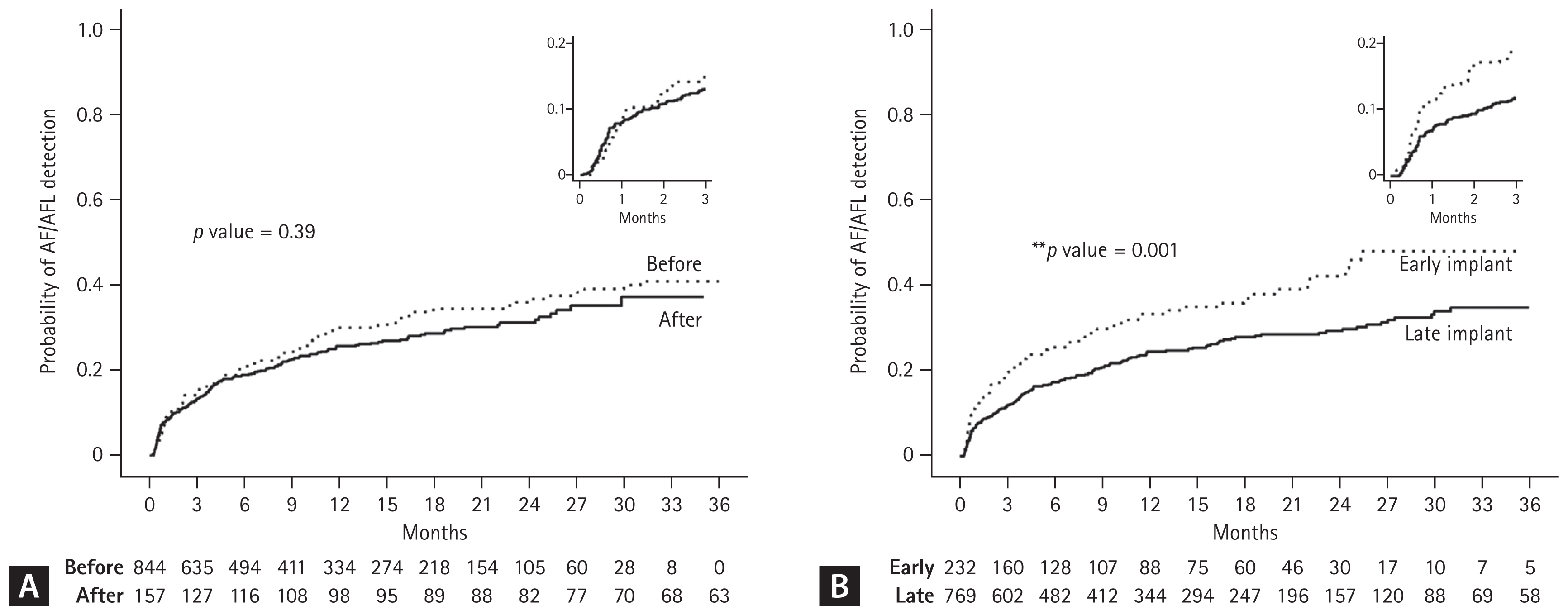
Table 1
Baseline characteristics of total patient
Table 2
Interrupted time-series analysis of the impact of the expanded reimbursement policy on the number of implantable loop recorder implantations per 4 months
Table 3
Characteristics of cryptogenic stroke patients before and after the reimbursement expansion for implantable loop recorder
REFERENCES
1. Edvardsson N, Frykman V, van Mechelen R, et al. Use of an implantable loop recorder to increase the diagnostic yield in unexplained syncope: results from the PICTURE registry. Europace 2011;13:262–269.

2. Lacunza-Ruiz FJ, Moya-Mitjans A, Martínez-Alday J, et al. Implantable loop recorder allows an etiologic diagnosis in onethird of patients. Results of the Spanish reveal registry. Circ J 2013;77:2535–2541.


3. Buck BH, Hill MD, Quinn FR, et al. Effect of implantable vs prolonged external electrocardiographic monitoring on atrial fibrillation detection in patients with ischemic stroke: the PER DIEM randomized clinical trial. JAMA 2021;325:2160–2168.


4. Sanna T, Diener HC, Passman RS, et al. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med 2014;370:2478–2486.


5. Kim HK, Song SO, Noh J, Jeong IK, Lee BW. Data configuration and publication trends for the Korean National Health Insurance and Health Insurance Review & Assessment database. Diabetes Metab J 2020;44:671–678.




6. Tsivgoulis G, Katsanos AH, Grory BM, et al. Prolonged cardiac rhythm monitoring and secondary stroke prevention in patients with cryptogenic cerebral ischemia. Stroke 2019;50:2175–2180.


7. Huang WY, Ovbiagele B, Hsieh CY, Lee M. Association between implantable loop recorder use and secondary stroke prevention: a meta-analysis. Open Heart 2022;9:e002034.



8. Jiang H, Tan SY, Wang JK, et al. A meta-analysis of extended ECG monitoring in detection of atrial fibrillation in patients with cryptogenic stroke. Open Heart 2022;9:e002081.



9. Skrebelyte-Strøm L, Rønning OM, Dahl FA, Steine K, Kjekshus H. Prediction of occult atrial fibrillation in patients after cryptogenic stroke and transient ischaemic attack: PROACTIA. Europace 2022;24:1881–1888.




10. Kim JG, Boo K, Kang CH, Kim HJ, Choi JC. Impact of neuroimaging patterns for the detection of atrial fibrillation by implantable loop recorders in patients with embolic stroke of undetermined source. Front Neurol 2022;13:905998.



11. Ahluwalia N, Graham A, Honarbakhsh S, et al. Contemporary practice and optimising referral pathways for implantable cardiac monitoring for atrial fibrillation after cryptogenic stroke. J Stroke Cerebrovasc Dis 2022;31:106474.


12. Samaan S, Kohli U, Nazeer B, et al. Detection of atrial fibrillation by implantable loop recorders following cryptogenic stroke: a retrospective study of predictive factors and outcomes. J Electrocardiol 2022;71:54–58.


13. Dulai R, Hunt J, Veasey RA, Biyanwila C, O’Neill B, Patel N. Immediate implantable loop recorder implantation for detecting atrial fibrillation in cryptogenic stroke. J Stroke Cerebrovasc Dis 2023;32:106988.


14. Navi BB, Singer S, Merkler AE, et al. Cryptogenic subtype predicts reduced survival among cancer patients with ischemic stroke. Stroke 2014;45:2292–2297.



15. Arboix A, Alió J. Cardioembolic stroke: clinical features, specific cardiac disorders and prognosis. Curr Cardiol Rev 2010;6:150–161.



16. Stöllberger C, Finsterer J. Detection of paroxysmal atrial fibrillation and patent foramen ovale in cryptogenic stroke. Eur J Neurol 2009;16:160–161.


17. Hart RG, Diener HC, Coutts SB, et al. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol 2014;13:429–438.


- TOOLS




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Supplement table 1
Supplement table 1 Print
Print



