 |
 |
| Korean J Intern Med > Volume 39(3); 2024 > Article |
|
Abstract
Background/Aims
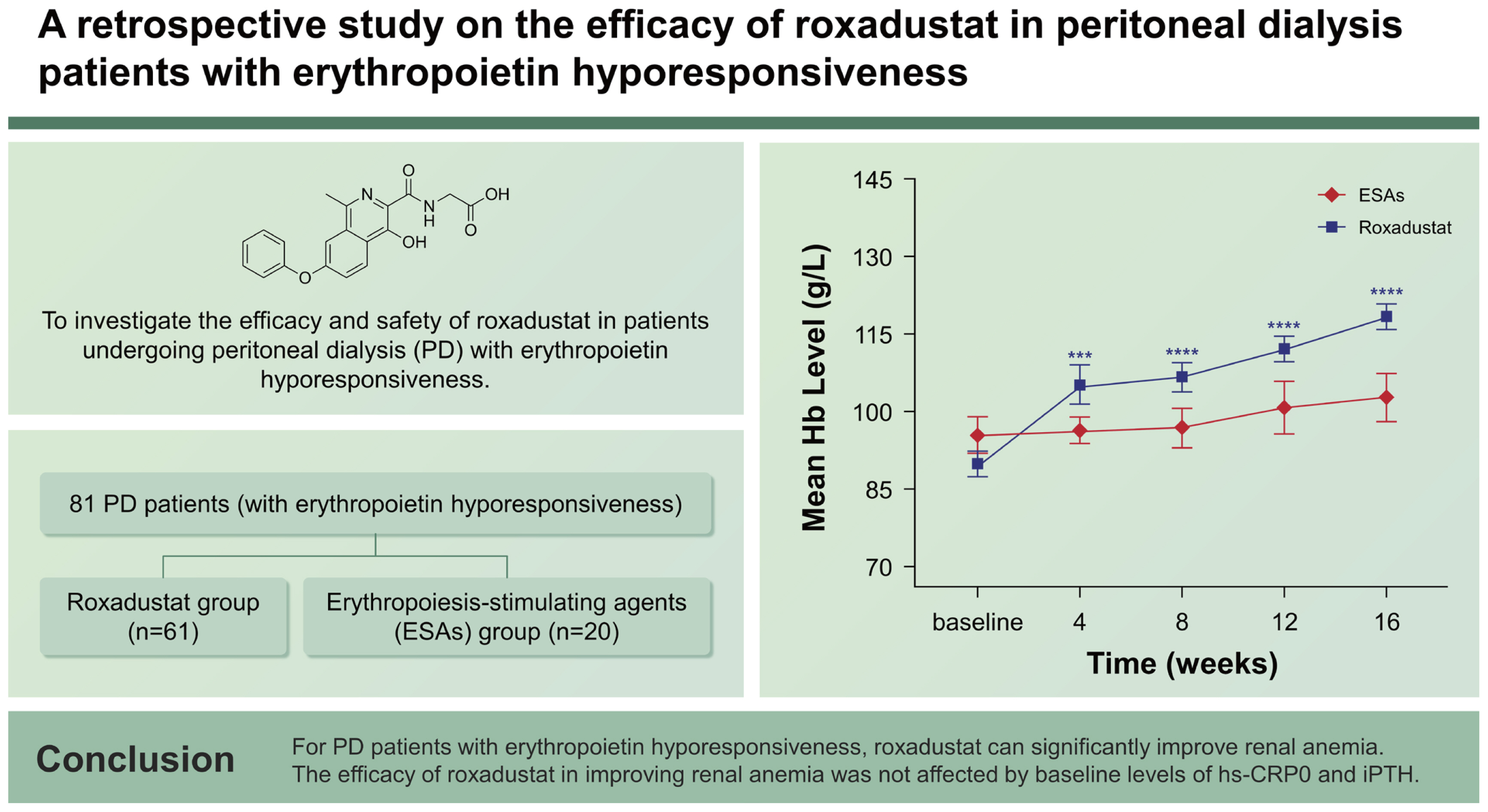
Roxadustat, an oral medication for treating renal anemia, is a hypoxia-inducible factor prolyl hydroxylase inhibitor used for regulating iron metabolism and promoting erythropoiesis. To investigate the efficacy and safety of roxadustat in patients undergoing peritoneal dialysis (PD) with erythropoietin hyporesponsiveness.
Methods
Single-center, retrospective study, 81 PD patients (with erythropoietin hyporesponsiveness) were divided into the roxadustat group (n = 61) and erythropoiesis-stimulating agents (ESAs) group (n = 20). Hemoglobin (Hb), total cholesterol, intact parathyroid hormone (iPTH), brain natriuretic peptide (BNP), related indicators of cardiac function and high-sensitivity C-reactive protein (hs-CRP) were collected. Additionally, adverse events were also recorded. The follow-up period was 16 weeks.
Results
The two groups exhibited similar baseline demographic and clinical characteristics. At baseline, the roxadustat group had a mean Hb level of 89.8 ± 18.9 g/L, while the ESAs group had a mean Hb level of 95.2 ± 16.0 g/L. By week 16, the Hb levels had increased to 118 ± 19.8 g/L (p < 0.05) in the roxadustat group and 101 ± 19.3 g/L (p > 0.05) in the ESAs group. The efficacy of roxadustat in improving anemia was not influenced by baseline levels of hs-CRP and iPTH. Cholesterol was decreased in the roxadustat group without statin use. An increase in left ventricular ejection fraction and stabilization of BNP were observed in the roxadustat group.
At present, chronic kidney disease (CKD) is still one of the most common diseases that threaten global human health. Its causes and complications are gradually diversified. How to better prevent and treat CKD is even more important. Renal anemia is one of the common complications, which not only affects the quality of life but also increases cardiovascular, cerebrovascular, and mortality risks [1,2]. Peritoneal dialysis (PD) is one of the kidney replacement therapies for patients with end-stage renal disease; it can mainly be operated by patients at home and can better protect residual renal function. PD patients account for about 11% of the global dialysis population [3], so it is particularly important to focus on the improvement of renal anemia in this special group.
Erythropoiesis-stimulating agents (ESAs) and iron supplements are the main treatments for renal anemia, but injection administration not only increases pain but also increases the risk of infection, thus reducing compliance [4]. In addition, some patients are not sensitive to ESAs [5], which makes it more difficult to correct anemia in PD patients with erythropoietin hyporesponsiveness. Not only that, some studies have found that ESAs are also closely related to patients’ cardiovascular diseases, elevated blood pressure, and stroke [6,7]. Moreover, the utilization and absorption of iron are also difficult to improve [8]. It can be seen that the traditional ways to improve renal anemia have obvious drawbacks, and it is necessary to find new ways to improve renal anemia.
Roxadustat is an oral medication for the treatment of renal anemia; it is a hypoxia-inducible factor prolyl hydroxylase inhibitor (HIF-PHI). It promotes erythropoiesis by inhibiting the HIF prolyl hydroxylase, as well as by mimicking the cellular response to hypoxia, thereby increasing the HIF activity [9]. Several domestic and foreign studies had proven the efficacy and safety of the drug for renal anemia. Among them, the study results of Akizawa et al. [10] showed that roxadustat could not only improve the Hb of PD patients but also help maintain Hb in the target range. This was the first clinical study on this drug for PD groups. Unfortunately, only 56 patients were included in the final study, and the topic of erythropoietin hyporesponsiveness was not involved. In addition, Chen et al. [11,12] conducted studies on hemodialysis (HD) groups and CKD patients who had not yet started dialysis, which also confirmed the effectiveness of roxadustat in improving renal anemia.
However, the current clinical studies on roxadustat mainly focus on HD and CKD patients who have not started dialysis, and there are few studies on this group of PD. In addition, it is worth noting that studies on roxadustat for the special group of PD combined with erythropoietin hyporesponsiveness are even rarer. This study aims to explore the efficacy of roxadustat in PD patients with erythropoietin hyporesponsiveness, and to objectively describe and analyze the adverse effects of roxadustat during actual clinical application.
We conducted a single-center retrospectivestudy, from the time of January 2019 to May 2023. All patients with erythropoietin hyporesponsiveness were divided into the roxadustat group and ESAs group according to drug use. PD patients (n = 61) who previously were receiving ESAs and switched to roxadustat were defined as the roxadustat group, and patients (n = 20) were defined as the ESAs group which they had an increased dose of ESAs or frequency of administration. All patients had adequate iron levels serum ferritin (SF) > 100 ng/mL, and transferrin saturation (TSAT) > 20% at the initial treatment stage. We used ESA resistance index (ERI) as an evaluation measure, which was calculated as the weight-adjusted weekly ESA dose divided by Hb concentration. When ERI ≥ 1 IU/kg/w/g/L, we considered the presence of erythropoietin hyporesponsiveness. In addition, these patients had been on adequate ESA treatment for more than three months, and their anemia-related causes had been actively treated, but their hemoglobin (Hb) was still below 110 g/L. The inclusion criteria were: (1) The patients were aged between 18 and 75 years old; (2) Patients with CKD associated with renal anemia underwent regular PD for more than 12 weeks. The exclusion criteria were: (1) Patients who were transferred to HD, kidney transplantation during the follow-up period; (2) Patients with other diseases causing anemia during the follow-up period, such as hematological disorders, hemorrhagic diseases resulting from various causes, and so on; (3) Patients who underwent blood transfusion during the follow-up period; (4) Patients with other serious chronic diseases, such as severe liver function impairment, severe heart failure, malignancy, serious infections, malignant hypertension. The specific screening scheme is shown in (Fig. 1). The study was approved by the Medical Ethics Committee of the Second Affiliated Hospital of Xuzhou Medical University (approval number: [2023]040601), and also completed Chinese Clinical Trial Registration (registration number: ChiCTR2300076409). This study was exempted from obtaining informed consents due to the retrospective character.
Only PD patients were included in the study, and the follow-up period was 16 weeks. All patients were given an initial therapeutic dose of 50–150 IU/kg per week of ESAs before the presence of erythropoietin hyporesponsiveness was determined, given by subcutaneous injection 1–3 times per week. In the roxadustat group, when we considered patients with erythropoietin hyporesponsiveness, we changed their anemia correction medication to roxadustat and discontinued ESAs. We determined the initial dose of roxadustat according to the drug instructions, giving 100 mg three times a week orally for subjects weighing 45–60 kg and 120 mg three times a week for subjects weighing ≥ 60 kg. The medication dose during the follow-up process was adjusted according to the comprehensive assessment of the current Hb level, Hb changes one month after taking the medication, the patient’s nutritional status, and iron metabolism level, making the target Hb level 110–130 g/L. In the ESAs group, we increased the dose of ESAs by 20 IU/kg each time or increased the frequency of administration as appropriate after the presence of erythropoietin hyporesponsiveness was determined, but the maximum dose of ESAs was not more than 2 times the stable dose. We referred to KDOQI clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target [13] and Clinical Practice Guidelines for Diagnosis and Treatment of renal anemia in China to determine the Hb target of this study between 110–130 g/L. During the whole treatment, we timely adjusted the dosage of both drugs, timely controlled inflammation, and followed up with patients monthly to ensure compliance. When SF ≤ 100 ng/mL or TSAT ≤ 20%, we started oral iron therapy, using ferrous succinate tablets 200 mg/d. The target value of SF was 100–500 ng/mL, and the target value of TSAT was 20–50%. Iron status was assessed at 1–3 months, and patients who did not reach the target value or could not tolerate it orally were switched to intravenous iron supplementation.
We collected basic patient information and laboratory test results from our hospital’s electronic medical record system. Regarding the adverse events of drugs, we collected relevant data through the electronic medical record system, and on the other hand, we supplemented relevant data through telephone follow-up. The primary indicators for adverse events were as follows: (1) hypertension, characterized by a substantial increase in blood pressure accompanied by a rapid elevation in Hb levels, which could be restored to baseline by reducing the dosage of the medication; (2) hyperkalemia, indicated by serum potassium levels exceeding 5.5 mmol/L; (3) hyperbilirubinemia, denoted by serum bilirubin levels surpassing 17.1 umol/L; (4) as well as other discomfort experienced after drug administration, including headache, insomnia, vomiting, diarrhea, and skin issues.
RStudio version 1.3 was used for statistical analysis and plotting. When the measurement data conformed to a normal distribution, it was represented by mean ± standard deviation, when it was not normally distributed, it was described by quartile M (P25, P75), statistical analysis was conducted by t-test or Wilcoxon-test. Tegorical variables are expressed as a percentage (%); statistical analysis was conducted by chi-squared test or Fisher’s exact test. The intra-group difference was compared by analysis of variance. Pearson correlation analysis was used to analyze its correlation. p < 0.05 indicated a statistically significant difference.
A total of 81 patients diagnosed with PD were included in the research, with 61 individuals in the roxadustat group and 20 individuals in the ESAs group. Among the participants, there were 52 males and 29 females, with an average age of 57.1 ± 15.2 years in the roxadustat group and 60.0 ± 11.3 years in the ESAs group. The average duration of PD was 18.0 ± 8.68 months for the roxadustat group and 21.6 ± 8.98 months for the ESAs group. The initial demographic and clinical characteristics of both groups did not exhibit any significant differences (p > 0.05). The basic information and the baseline values of laboratory indicators are shown in (Table 1).
The baseline Hb levels of roxadustat and ESAs groups were 89.8 ± 18.9 g/L and 95.2 ± 16.0 g/L, respectively, with no significant difference between groups (p = 0.218). And over time, the mean Hb levels of roxadustat group increased to 105 ± 29.5 g/L at week 4, 107 ± 21.8 g/L at week 8, 112 ± 20.3 g/L at week 12, and 118 ± 19.8 g/L at week 16, respectively, showing significant differences from baseline values at all time nodes (p < 0.05). However, in the ESAs group, the mean Hb value was 96.4±11.4 g/L at week 4, 96.8 ± 17.1 g/L at week 8, 98.2 ± 19.1 g/L at week 12, and 101 ± 19.3 g/L at week 16, all of which did not change significantly from baseline (p > 0.05) (Fig. 2).
The laboratory of our hospital determined that the upper limit of the normal value for hs-CRP is 5 mg/L. We categorized the patients into two subgroups based on their hs-CRP baseline levels: hs-CRP < 5 mg/L and hs-CRP ≥ 5 mg/L. We then compared the changes in Hb levels within each subgroup. The results indicated that in the roxadustat group, both subgroups (hs-CRP < 5 mg/L and hs-CRP ≥ 5 mg/L) had significantly higher Hb values at week 16 compared to their baseline values (122 ± 22.4 g/L vs. 93.8 ± 17.3 g/L and 114 ± 15.8 g/L vs. 85.3 ± 19.9 g/L, respectively), with statistical significance (p < 0.05) (Fig. 3A). However, in the ESAs group, there were no significant changes in Hb values within the two subgroups at week 16 compared to their baseline values p > 0.05) (Fig. 3B).
Additionally, we referred to the 2003 K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. According to the guideline’s suggested target for iPTH, we divided iPTH into three subgroups: < 150 pg/mL, 150–300 pg/mL, and > 300 pg/mL. We then analyzed the changes in Hb values within each subgroup. The results showed that in the roxadustat group, Hb values at week 16 were significantly higher than baseline values in all three subgroups (123 ± 14.0 g/L vs. 93.1 ± 17.4 g/L, 117 ± 23.1 g/L vs. 85.4 ± 18.1 g/L, and 115 ± 20.5 g/L vs. 92.1 ± 21.3 g/L), with statistical significance (p < 0.05) (Fig. 3C). However, no significant differences were observed within the subgroups of the ESAs group (Fig. 3D).
We divided the roxadustat group and ESAs group into statin and non-statin subgroups based on whether or not they were using statins, and compared changes in total cholesterol levels, low-density lipoprotein (LDL), and high-density lipoprotein (HDL) over time. The results showed that total cholesterol in the roxadustat statin subgroup decreased significantly at weeks 12 and 16 compared with the baseline values (3.78 ± 0.951 mmol/L vs. 4.93 ± 1.92 mmol/L and 3.64 ± 0.799 mmol/L vs. 4.93 ± 1.92 mmol/L). The specific values at each time node are shown in Figure 4A. The p values at week 12, and week 16 compared with the baseline were 0.048 and 0.011, respectively. Similarly, total cholesterol decreased significantly from baseline at weeks 12 and 16 in the non-statin subgroup of roxadustat (3.68 ± 1.04 mmol/L vs. 4.31 ± 0.833 mmol/L and 3.62 ± 1.06 mmol/L vs. 4.31 ± 0.833 mmol/L), p values were 0.031 and 0.013 compared with the baseline at week 12 and week 16, respectively. However, in the ESAs group, only the statin subgroup had a significant decrease in total cholesterol level at week 16 compared with the baseline value (3.56 ± 0.768 mmol/L vs. 4.80 ± 1.37 mmol/L, p = 0.049), and the total cholesterol value at each time node was listed in detail in Figure 4B.
LDL values in the non-statins and statins subgroups of the roxadustat group at week 16 were significantly lower than the baseline value (2.38 ± 1.37 mmol/L vs. 3.39 ± 1.48 mmol/L and 2.37 ± 1.19 mmol/L vs. 3.56 ± 1.80 mmol/L), with statistically significant differences (p = 0.024 and 0.04), and the specific values at each time node were shown in Figure 4C. However, in the ESAs group, only the statin subgroup had a significant decrease in LDL level at week 16 compared with the baseline value (2.43 ± 0.925 mmol/L vs. 3.64 ± 1.09 mmol/L, p = 0.029), and the LDL value at each time node was listed in detail in Figure 4D. In relation to HDL, the HDL values within the subgroups of both the roxadustat and ESA groups remained relatively stable throughout the study period, with no statistically significant differences (p > 0.05), as illustrated in Figure 4E and Figure 4F.
A statistical analysis was conducted to examine the impact of roxadustat on cardiac function. The evaluation indicators used in this study included brain natriuretic peptide (BNP), cardiac ejection fraction, left ventricular end-systolic dimension (LVESD), and left ventricular end-diastolic dimension (LVEDD). In the group receiving roxadustat, there was a gradual increase in left ventricular ejection fraction as anemia improved. This increase was significant compared to the baseline measurements at week 12 and week 16 (54.1 ± 4.4% vs. 49.4 ± 7.08% and 58.5 ± 2.74% vs. 49.4 ± 7.08%), with statistically significant differences (p < 0.0001 and < 0.0001). However, no significant change in left ventricular ejection fraction was observed in the group receiving ESAs. The values of left ventricular ejection fraction at each time point for both groups are presented in Figure 5A. The BNP values for both groups did not show any significant changes throughout the follow-up period (p > 0.05), as depicted in Figure 5B.
Although there were no significant changes in LVESD values in both groups throughout the follow-up period, it is important to note that in the roxadustat group, LVESD gradually decreased as anemia improved and nearly approached statistical significance compared to baseline at week 16 (33.0 ± 4.80 mm vs. 35.3 ± 4.72 mm, p = 0.056). The specific LVESD values at each time point for both groups are presented in Figure 5C. Similarly, the LVEDD values did not show significant changes during the entire follow-up period for both groups, but there was an overall downward trend in the roxadustat group, and a near statistical difference compared to baseline was observed at week 16 (49.2 ± 4.25 mm vs. 51.0 ± 2.83 mm, p = 0.057). The specific values at each time point are displayed in Figure 5D.
During the entire follow-up period, one patient in the roxadustat group died of severe peritonitis at day 65, but we did not consider this to be due to adverse drug reactions. No serious thromboembolic disease and cardiovascular adverse events occurred. It is worth noting that three patients developed hyperbilirubinemia and returned to normal after appropriate treatment, but we do not have sufficient evidence to suggest that it was caused by adverse reactions to roxadustat. Five individuals encountered a swift elevation in Hb levels and a notable rise in blood pressure from their initial state, and subsequently, their blood pressure gradually reverted back to the initial levels following a reduction in drug dosage. Four patients exhibited symptoms of headaches, which subsided after the reduction in dosage. A limited number of patients experienced insomnia, vomiting, diarrhea, and pruritic skin; however, these symptoms vanished after receiving treatment. In general, there was no substantial disparity in the occurrence of unfavorable events between the roxadustat and ESA groups. The adverse events are shown in (Table 2).
Renal anemia is a common complication of CKD, and traditional treatment options include ESAs and iron agents, but thorny problems such as erythropoietin hyporesponsiveness can also come with it. There were only a few previous conducted clinical studies on the efficacy of roxadustat in PD patients with erythropoietin hyporesponsiveness, but the sample size was small (the largest one included 55 patients) and the study content was relatively simple [14,15]. In the current study, we aimed to discuss this issue in depth.
The findings of our study indicate that roxadustat has a significant impact on improving the anemia status of patients who are unresponsive to erythropoietin. However, adjusting the dose or frequency of administration of ESAs did not yield a definitive effect. This discovery will provide new insights into the treatment of renal anemia in PD patients who have erythropoietin hyporesponsiveness. Previous studies have also demonstrated that roxadustat can inhibit hepcidin and play a role in the entire process of iron metabolism, including its absorption, transport, utilization, and recycling [16–18]. The baseline values of serum SF and TSAT in both groups are presented in Table 1. Our iron supplementation regimen has been described previously. It is evident that for PD patients with erythropoietin hyporesponsiveness, roxadustat has a superior effect in correcting anemia compared to ESAs, even at the same level of iron metabolism. Unfortunately, due to limitations in our testing equipment, routine assessment of hepcidin was not conducted at our center.
Inflammation promotes the development and progression of anemia in CKD patients through multiple pathways, including inhibiting erythroid hyperplasia, enhancing erythrophagocytosis, inhibiting erythropoietin production, and increasing liver secretion of hepcidin [19]. Previous studies have shown that the effect of roxadustat on anemia in both HD and PD patients is not affected by inflammatory status, which is assessed by hs-CRP [16]. Our study revealed a significant increase in Hb levels among PD patients with erythropoietin hyporesponsiveness in both the hs-CRP < 5 mg/L and hs-CRP ≥ 5 mg/L subgroups within the roxadustat group. Conversely, no statistical change was observed in the ESAs group, aligning with the outcomes of previous research studies. These findings suggest that roxadustat is a better choice than ESAs for patients with renal anemia in a microinflammatory state. It is worth noting that in addition to the inflammatory state, the increase of iPTH will also lead to the poor effect of ESAs in improving anemia [20,21]. The specific mechanisms include: the direct effect of increased iPTH is to inhibit hematopoietic progenitor cells, reduce the production of erythropoietin, and shorten the life of red blood cells. The indirect effect is to cause myelofibrosis and increase alkaline phosphatase. These conditions can lead to erythropoietin hyporesponsiveness and aggravate renal anemia [22]. Interestingly, however, we found that the efficacy of roxadustat in improving anemia was not associated with baseline iPTH levels, which has not been reported in previous clinical studies on the efficacy of roxadustat in PD patients, and this is the innovation of our study.
Previous studies have shown that roxadustat can reduce total cholesterol in PD patients [17], but the specific mechanism is not yet understood, which may be related to the acetyl coenzyme A and the degradation of 3-hydroxy-3-methyl-glutaryl coenzyme A reductase, which is the first step of cholesterol synthesis required former enzyme [23,24]. It is worth noting that different HIF-PHI has different effects on lipid metabolism. For example, a clinical study conducted on HD patients in Japan found that daprodustat had similar cholesterol-lowering effects [25], but a study in stage 3–4 CKD patients in the United States found that vadadustat had no effect on cholesterol and triglycerides [26]. Our results also found that roxadustat may have a cholesterol-lowering effect, which is also beneficial for cardiovascular disease prevention in patients with CKD.
The probability of anemia in patients with CKD increases with the progression of the disease, the prevalence of anemia in patients with stage 5 CKD is 90.2%, and the prevalence of anemia after dialysis is as high as 98% [27]. The study of Akaishi et al. [28] found that the left ventricular mass index (LVMI) of patients with high Hb level (110 g/L ≤ Hb ≤ 120 g/L) was ≥ 2 times lower than that of patients with low Hb level (100 g/L ≤ Hb ≤ 110 g/L), where LVMI is an indicator to evaluate left ventricular function, indicating that the control of Hb within the target range is conducive to cardiac function. Moreover, the BNP of those whose Hb reached the target (110 g/L ≤ Hb ≤ 130 g/L) was 50 pg/mL lower than that of those whose Hb did not meet the standard [29], indicating that Hb reaching the standard was conducive to controlling the stability of BNP. Our results also showed that, the left ventricular ejection fraction gradually increased with the improvement of anemiain the roxadustat group, and the increase in the ejection fraction from the 12th week was statistically significant, which may be because the average Hb in this group of patients did not reach the target until the 12th week. There was no statistical change in BNP during the whole course of treatment, because the mean left ventricular ejection fraction was > 50% from week 4, and the anemia was improved from week 4. At the 16th week, the roxadustat group exhibited a decrease in both LVESD and LVEDD measurements compared to the baseline, nearly approached statistical significance. If the follow-up period was extended and the sample size was increased, it is possible that a significant difference could be observed, aligning with the direction of our follow-up. Conversely, no statistically significant findings were observed in the ESAs group. These results imply that roxadustat might offer potential benefits in enhancing cardiac function among PD patients who exhibit erythropoietin hyporesponsiveness.
At present, there are different opinions on the effect of roxadustat on serum potassium. Some studies showed that roxadustat can increase serum potassium [11,12], and some studies showed that there was no statistical difference in serum potassium between roxadustat group and control group [30–32]. Our results showed that a total of 5 (8.2%) patients developed hyperkalemia, which was not significantly different from the ESAs group. In addition, several studies have found an increased risk of thromboembolic events with roxadustat, however, we did not find this in our study, which may be related to our relatively short follow-up period and relatively small study sample [30,33–36].
Our study has several obvious advantages. First of all, most of the studies about roxadustat focused on HD or non-dialysis patients with CKD and relatively few studies on PD patients. Second, there are very few studies on roxadustat in patients with low erythropoietin responsiveness undergoing PD. Compared with previous studies, our study enrolled more individuals and the content of the study was more comprehensive. Finally, this is the first clinical study to investigate the impact of baseline levels of iPTH on the efficacy of roxadustat and ESAs in treating renal anemia. This will bring new therapeutic directions and ideas for the treatment of anemia patients undergoing PD with erythropoietin hyporesponsiveness.
Overall, roxadustat can significantly improve renal anemia in PD patients with erythropoietin hyporesponsiveness. In addition, the effect of roxadustat on anemia was not affected by baseline hs-CRP and iPTH values. Finally, roxadustat has positive effects on lowering total cholesterol and improving heart function in PD patients.
This was a single-center study with a relatively small sample size and a relatively short follow-up time. Since there have been many previous articles on roxadustat and iron metabolism related indicators, our study did not carry out more research on iron metabolism indicators.
1. Roxadustat can significantly improve renal anemia in PD patients with erythropoietin hyporesponsiveness.
2. The effect of roxadustat on anemia was not affected by baseline hs-CRP and iPTH values.
3. Roxadustat has positive effects on lowering total cholesterol and improving heart function in PD patients.
Acknowledgments
We would like to express our sincere thanks to every member of our research team for their hard-work and the strong support provided by the hospital.
Notes
CRedit authorship contributions
Jie Liu: conceptualization, methodology, resources, writing - original draft, funding acquisition; Shuang Li: investigation, data curation; Fan Yang: investigation, data curation; Tianyu Li: investigation, data curation; Rui Li: software; Yousuf Waheed: writing - review & editing; Chen Meng: software; Shulin Li: funding acquisition; Kun Liu: software; Yanshan Tong: software; Haisheng Xu: formal analysis; Chuankuo Tian: software; Xinglei Zhou: conceptualization, supervision, project administration, funding acquisition
Figure 1
Patient flow diagram. PD, peritoneal dialysis; HD, hemodialysis; ESA, erythropoiesis-stimulating agent; ERI, ESA resistance index.

Figure 2
Mean hemoglobin levels of Roxadustat and ESAs groups over time. Hb, hemoglobin; ESA, erythropoiesis-stimulating agent. ***p < 0.001 versus baseline level, ****p < 0.0001 versus the corresponding baseline level.
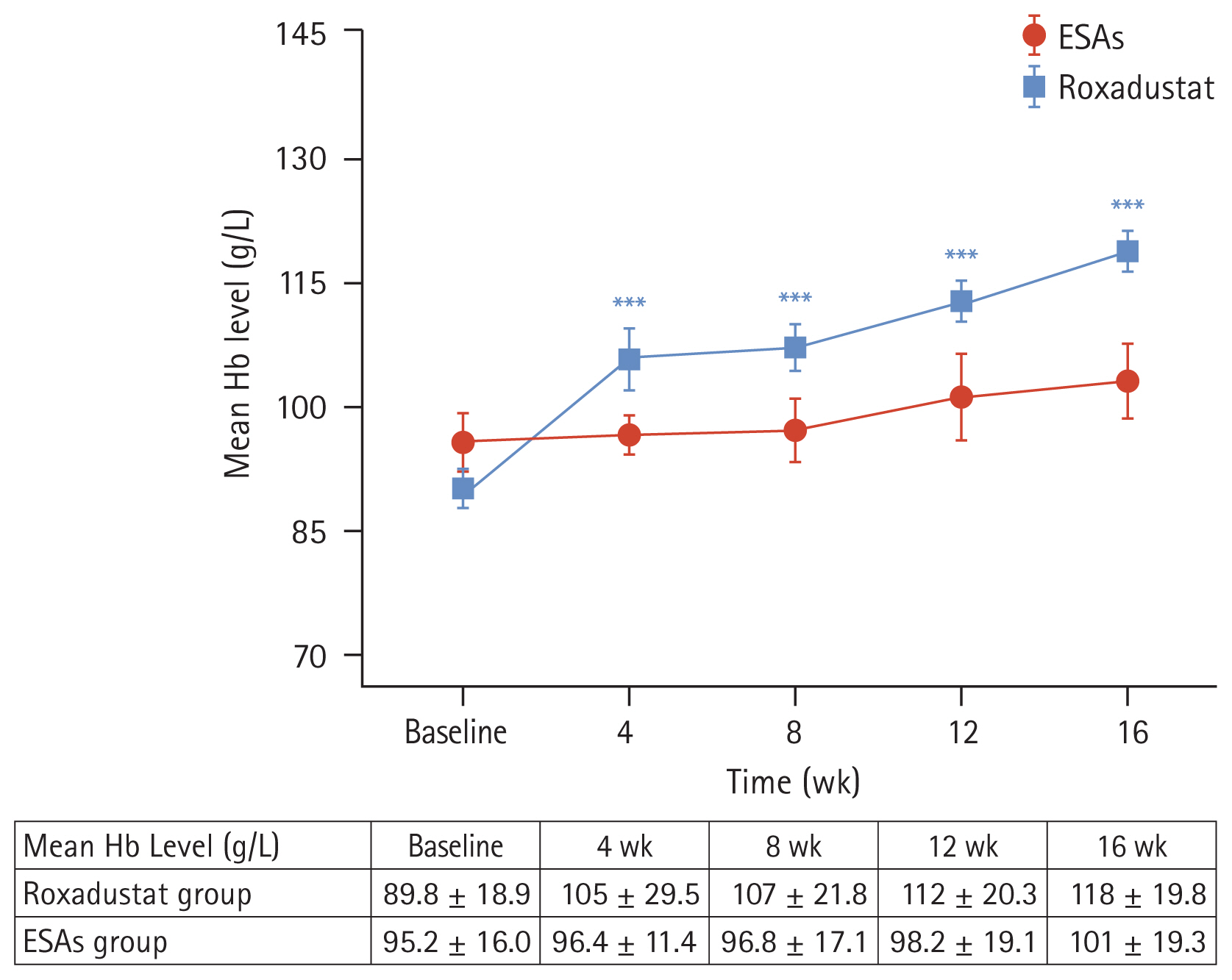
Figure 3
Mean hemoglobin levels over time in the roxadustat group (A) and ESAs group (B) according to the hs-CRP subgroup. Mean hemoglobin levels over time in the roxadustat group (C) and ESAs group (D) according to the iPTH subgroup. Hb, hemoglobin; hs- CRP, high-sensitivity C-reactive protein; ESA, erythropoiesis-stimulating agent; iPTH, intact parathyroid hormone. *p < 0.05 versus the corresponding baseline level, **p < 0.01 versus the corresponding baseline level, ***p < 0.001 versus the corresponding baseline level, ****p < 0.0001 versus the corresponding baseline level.
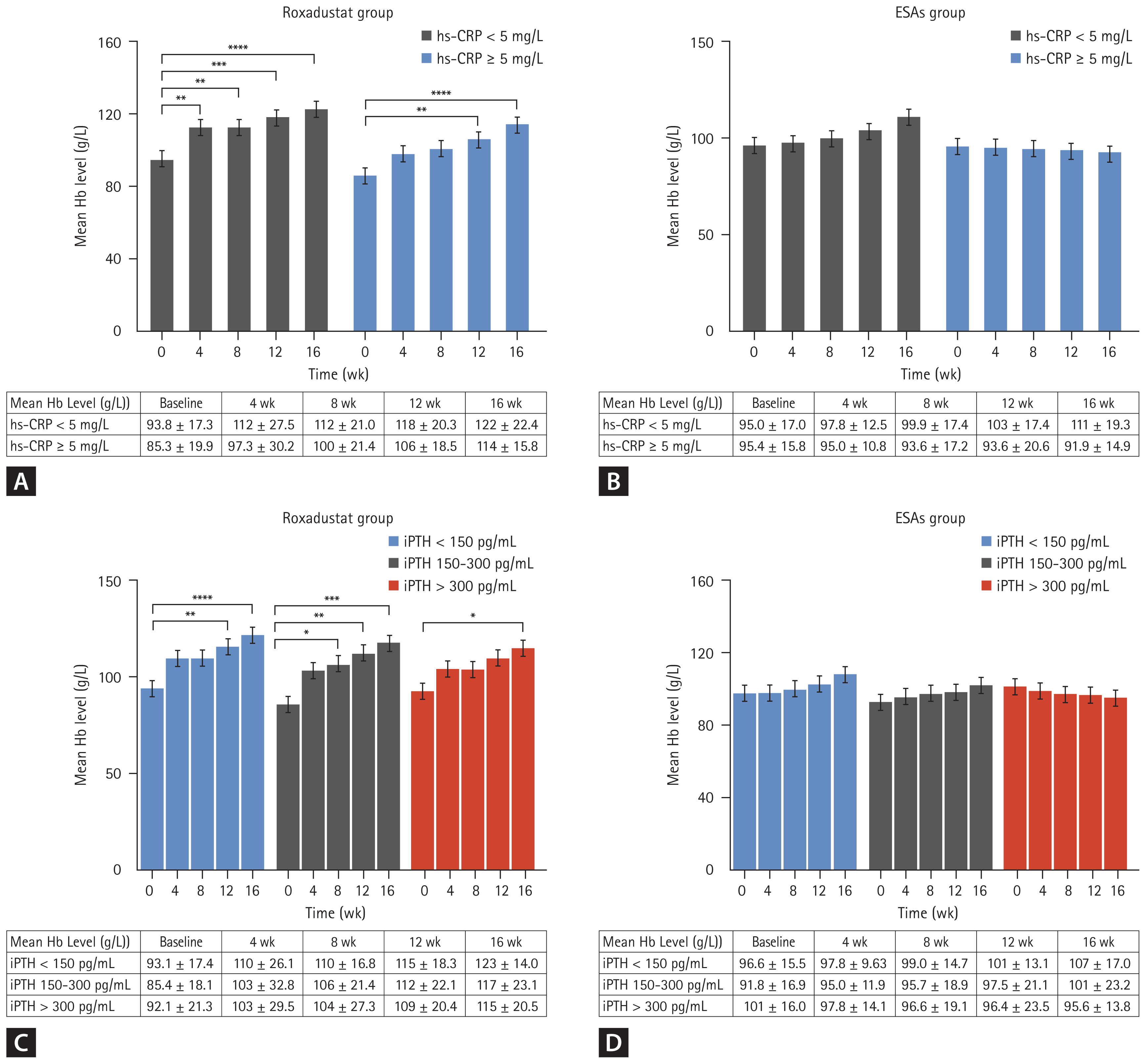
Figure 4
Mean total cholesterol levels over time in the roxadustat group (A) and ESAs group (B) according to the statins subgroup. Mean LDL-cholesterol levels over time in the roxadustat group (C) and ESAs group (D) according to the statins subgroup. Mean HDL-cholesterol levels over time in the roxadustat group (E) and ESAs group (F) according to the statins subgroup. ESA, erythropoiesis-stimulating agent; LDL, low-density lipoprotein; HDL, high-density lipoprotein. *p < 0.05 versus the corresponding baseline level.
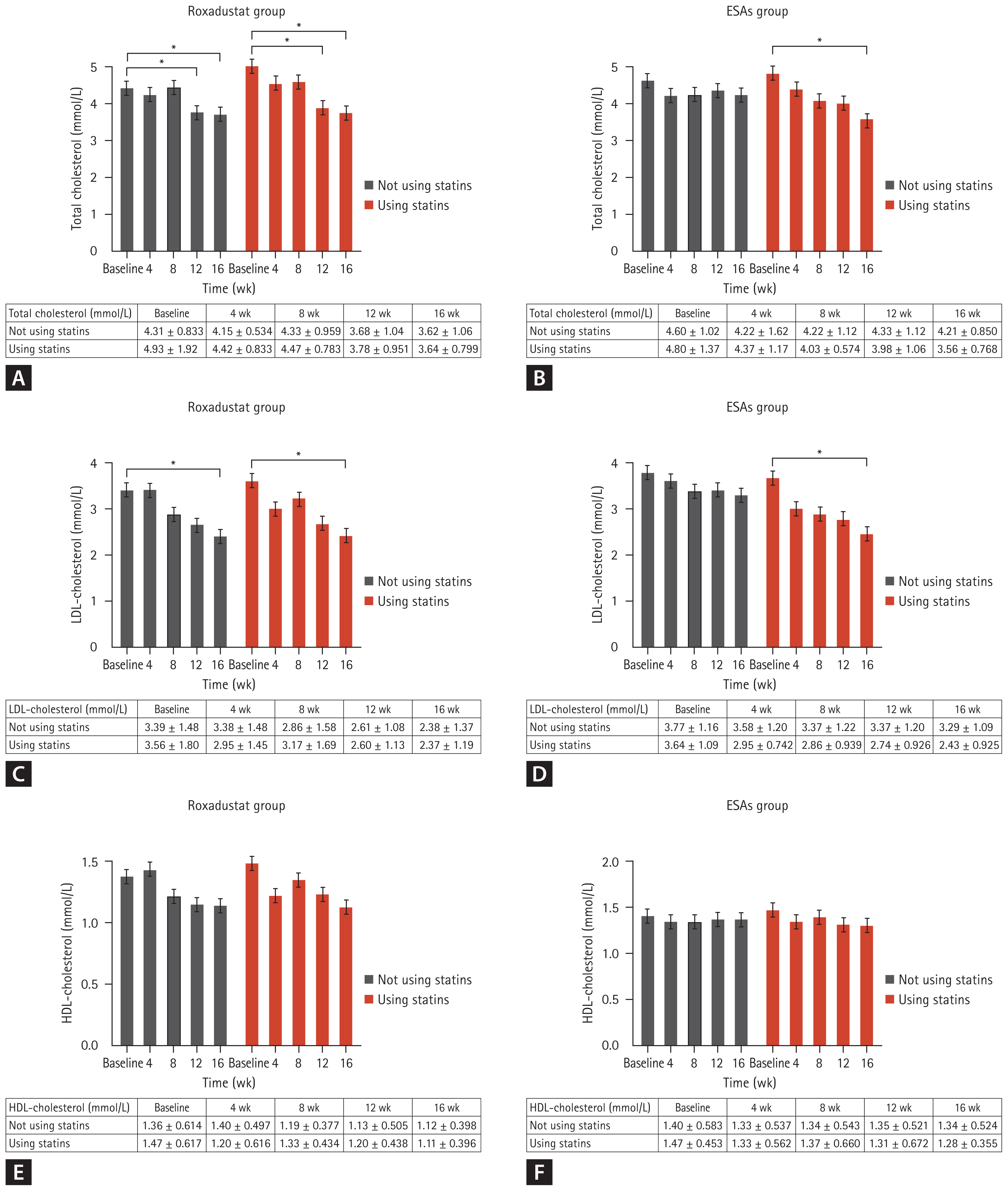
Figure 5
(A) Changes of left ventricular ejection fraction over time in Roxadustat and ESAs groups. (B) Changes of BNP over time in Roxadustat and ESAs groups. (C) Changes of LVESD over time in Roxadustat and ESAs groups. (D) Changes of LVEDD over time in Roxadustat and ESAs groups. ESA, erythropoiesis-stimulating agent; BNP, brain natriuretic peptide; LVESD, left ventricular end- systolic dimension; LVEDD, left ventricular end-diastolic dimension. ****p < 0.0001 versus the corresponding baseline level.
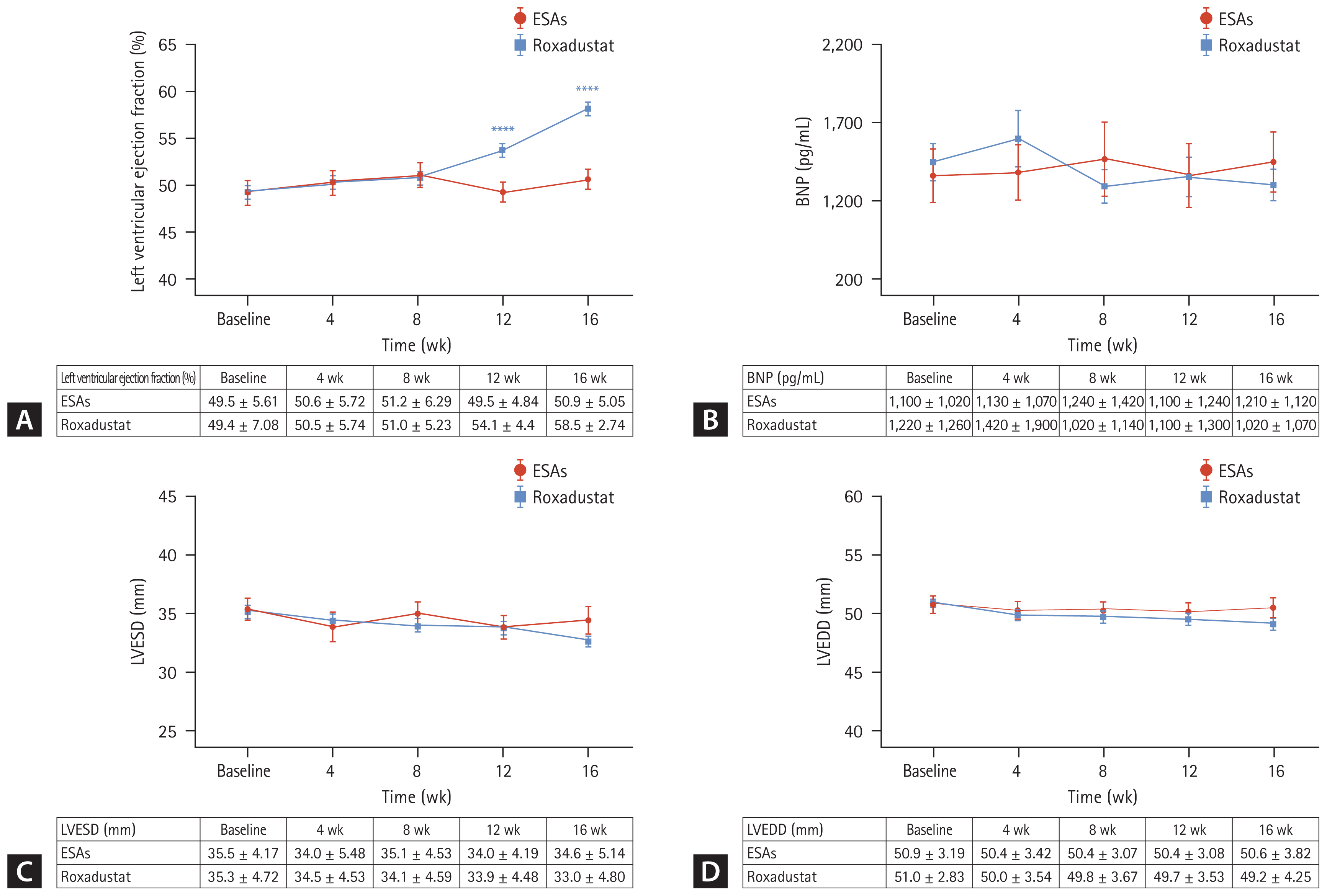
Table 1
Main baseline characteristics of patients included in the study
Continuous variables are expressed as mean ± standard deviation and categorical variables are represented as number (%).
ESA, erythropoiesis-stimulating agent; CKD, chronic kidney disease; CAPD, continuous ambulatory peritoneal dialysis; Kt/V, urea clearance; LDL, low-density lipoprotein; HDL, high-density lipoprotein; hs-CRP, high-sensitivity C-reactive protein; iPTH, intact parathyroid hormone; LVEDD, left ventricular end-diastolic dimension; LVESD, left ventricular end-systolic dimension.
Table 2
Summary of adverse events after 16 weeks
REFERENCES
1. Toft G, Heide-Jørgensen U, van Haalen H, et al. Anemia and clinical outcomes in patients with non-dialysis dependent or dialysis dependent severe chronic kidney disease: a Danish population-based study. J Nephrol 2020;33:147–156.




2. Li S, Foley RN, Collins AJ. Anemia, hospitalization, and mortality in patients receiving peritoneal dialysis in the United States. Kidney Int 2004;65:1864–1869.


3. Li PK, Chow KM, Van de Luijtgaarden MW, et al. Changes in the worldwide epidemiology of peritoneal dialysis. Nat Rev Nephrol 2017;13:90–103.



4. Johnson DW, Pollock CA, Macdougall IC. Erythropoiesis-stimulating agent hyporesponsiveness. Nephrology (Carlton) 2007;12:321–330.

5. Ogawa T, Nitta K. Erythropoiesis-stimulating agent hyporesponsiveness in end-stage renal disease patients. Contrib Nephrol 2015;185:76–86.


6. Singh AK, Carroll K, McMurray JJV, et al. Daprodustat for the treatment of anemia in patients not undergoing dialysis. N Engl J Med 2021;385:2313–2324.


7. Koulouridis I, Alfayez M, Trikalinos TA, Balk EM, Jaber BL. Dose of erythropoiesis-stimulating agents and adverse outcomes in CKD: a metaregression analysis. Am J Kidney Dis 2013;61:44–56.



8. Del Vecchio L, Minutolo R. ESA, iron therapy and new drugs: are there new perspectives in the treatment of anaemia? J Clin Med 2021;10:839.



9. Gupta N, Wish JB. Hypoxia-inducible factor prolyl hydroxylase inhibitors: a potential new treatment for anemia in patients with CKD. Am J Kidney Dis 2017;69:815–826.

10. Akizawa T, Yamaguchi Y, Otsuka T, Reusch M. A phase 3, multicenter, randomized, two-arm, open-label study of inter-mittent oral dosing of roxadustat for the treatment of anemia in Japanese erythropoiesis-stimulating agent-naïve chronic kidney disease patients not on dialysis. Nephron 2020;144:372–382.




11. Chen N, Hao C, Liu BC, et al. Roxadustat treatment for anemia in patients undergoing long-term dialysis. N Engl J Med 2019;381:1011–1022.


12. Chen N, Hao C, Peng X, et al. Roxadustat for anemia in patients with kidney disease not receiving dialysis. N Engl J Med 2019;381:1001–1010.

13. KDOQI. KDOQI Clinical Practice Guideline and Clinical Practice Recommendations for anemia in chronic kidney disease: 2007 update of hemoglobin target. Am J Kidney Dis 2007;50:471–530.

14. Dai S, Chen Y, Hao C, et al. Addition of roxadustat to erythropoiesis-stimulating agent (ESA) effectively corrects ESA-hyporesponsive anaemia in patients on peritoneal dialysis. J Clin Pharm Ther 2022;47:1525–1530.

15. Chen J, Li Z, Zhang H, et al. A prospective, self-controlled pilot study of the efficacy of roxadustat for erythropoietin hyporesponsiveness in patients requiring chronic ambulatory peritoneal dialysis. J Ren Nutr 2022;32:595–604.


16. Besarab A, Chernyavskaya E, Motylev I, et al. Roxadustat (FG-4592): correction of anemia in incident dialysis patients. J Am Soc Nephrol 2016;27:1225–1233.

17. Hou YP, Mao XY, Wang C, et al. Roxadustat treatment for anemia in peritoneal dialysis patients: a randomized controlled trial. J Formos Med Assoc 2022;121:529–538.


18. Hirai K, Nonaka H, Ueda M, et al. Effects of roxadustat on the anemia and iron metabolism of patients undergoing peritoneal dialysis. Front Med (Lausanne) 2021;8:667117.



19. Zarychanski R, Houston DS. Anemia of chronic disease: a harmful disorder or an adaptive, beneficial response? CMAJ 2008;179:333–337.



20. Tanaka M, Komaba H, Fukagawa M. Emerging association between parathyroid hormone and anemia in hemodialysis patients. Ther Apher Dial 2018;22:242–245.



21. Gaweda AE, Goldsmith LJ, Brier ME, Aronoff GR. Iron, inflammation, dialysis adequacy, nutritional status, and hyperparathyroidism modify erythropoietic response. Clin J Am Soc Nephrol 2010;5:576–581.



22. Kuragano T, Kitamura K, Matsumura O, et al. ESA hyporesponsiveness is associated with adverse events in maintenance hemodialysis (MHD) patients, but not with iron storage. PLoS One 2016;11:e0147328.



23. Nguyen AD, McDonald JG, Bruick RK, DeBose-Boyd RA. Hypoxia stimulates degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase through accumulation of lanosterol and hypoxia-inducible factor-mediated induction of insigs. J Biol Chem 2007;282:27436–27446.


24. Hwang S, Nguyen AD, Jo Y, Engelking LJ, Brugarolas J, De-Bose-Boyd RA. Hypoxia-inducible factor 1α activates insulin-induced gene 2 (Insig-2) transcription for degradation of 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase in the liver. J Biol Chem 2017;292:9382–9393.



25. Akizawa T, Tsubakihara Y, Nangaku M, et al. Effects of daprodustat, a novel hypoxia-inducible factor prolyl hydroxylase inhibitor on anemia management in Japanese hemodialysis subjects. Am J Nephrol 2017;45:127–135.



26. Martin ER, Smith MT, Maroni BJ, Zuraw QC, deGoma EM. Clinical trial of vadadustat in patients with anemia secondary to stage 3 or 4 chronic kidney disease. Am J Nephrol 2017;45:380–388.




27. Li Y, Shi H, Wang WM, et al. Prevalence, awareness, and treatment of anemia in Chinese patients with nondialysis chronic kidney disease: first multicenter, cross-sectional study. Medicine (Baltimore) 2016;95:e3872.


28. Akaishi M, Hiroe M, Hada Y, Suzuki M, Tsubakihara Y, Akizawa T, KRN321 Study Group. Effect of anemia correction on left ventricular hypertrophy in patients with modestly high hemoglobin level and chronic kidney disease. J Cardiol 2013;62:249–256.

29. Suzuki M, Hada Y, Akaishi M, et al. Effects of anemia correction by erythropoiesis-stimulating agents on cardiovascular function in non-dialysis patients with chronic kidney disease. Int Heart J 2012;53:238–243.


30. Fishbane S, Pollock CA, El-Shahawy M, et al. Roxadustat Versus epoetin alfa for treating anemia in patients with chronic kidney disease on dialysis: results from the randomized phase 3 ROCKIES study. J Am Soc Nephrol 2022;33:850–866.



31. Charytan C, Manllo-Karim R, Martin ER, et al. A randomized trial of roxadustat in anemia of kidney failure: SIERRAS study. Kidney Int Rep 2021;6:1829–1839.



32. Coyne DW, Roger SD, Shin SK, et al. Roxadustat for CKD-related anemia in non-dialysis patients. Kidney Int Rep 2020;6:624–635.



34. Provenzano R, Shutov E, Eremeeva L, et al. Roxadustat for anemia in patients with end-stage renal disease incident to dialysis. Nephrol Dial Transplant 2021;36:1717–1730.



-
METRICS

-
- 0 Crossref
- 0 Scopus
- 27 View
- 54 Download
- Related articles
-
The efficacy of denosumab in Korean male patients with osteoporosis2022 September;37(5)



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print


