 |
 |
| Korean J Intern Med > Volume 22(4); 2007 > Article |
|
Abstract
Combined hepatocellular-cholangiocarcinoma is a rare form of primary liver cancer showing features of both hepatocellular and biliary epithelial differentiation. We report here on a case with collision tumor, which apparently was the coincidental occurrence of both hepatocellular carcinoma and cholangiocarcinoma underlying schistosomiasis. A 39-year-old-Philippine female was transferred to our hospital for evaluation of a liver mass that was found on ultrasonography at a local hospital. HBsAg and Anti-HCV were negative and serum alpha-fetoprotein (AFP) level was normal. The tumor mass was histologically diagnosed as adenocarcinoma by sono-guided biopsy before the operation. Partial lobectomy was performed and we histologically identified the concurrent occurrence of hepatocellular carcinoma and cholangiocarcinoma, (a "collision type carcinoma").
More than 80% of primary liver cancers are hepatocellular carcinomas (HCCs), and these tumors usually occur in livers that are cirrhotic due to chronic hepatitis B or C viral infections, a high alcohol intake or hemochromatosis1). The second most frequent primary tumors of the liver are cholangiocarcinomas (CC); they account for about 15% of primary liver cancers, and they usually occur in fibropolycystic disease, during a chronic inflammatory process of the bile ducts and after exposure to thorotrast or steroids2-5). A less frequent primary liver tumor type is the hepatocellular carcinoma-cholangiocarcinoma (HCC-CC) which shows dual hepatocellular and biliary epithelial differentiations in the same tumor, yet these have rarely been reported on in the English and Korean literature6). We report here on a case of collision tumor in the liver with schistosome infection, and there was metastatic lesion in the lymph node specimen.
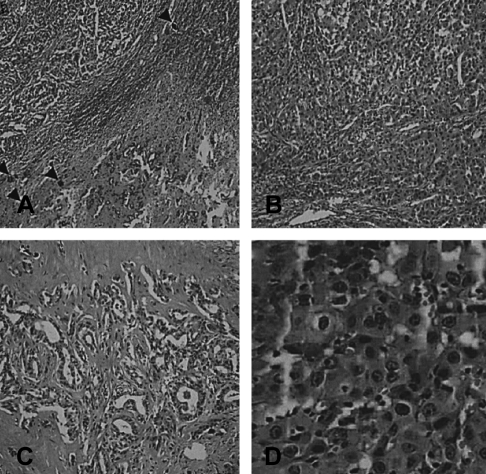
A 39-year-old Philippine female was transferred to our hospital for evaluation of a liver mass that was observed on abdominal ultrasonography at a local hospital. She'd experienced right upper quadrant pain for the previous 2 months. The patient had no history of diabetes, hepatitis or tuberculosis. She had no history of alcohol drinking or smoking. On admission, the body temperature was 37℃, the pulse rate was 66 per minute, the respiration rate was 20 per minute and the blood pressure was 100/60 mmHg. Physical examinations showed anicteric sclerae with non-anemic conjunctivae and no cervical lymphadenopathy. Abdominal palpation revealed no palpable mass. Laboratory examinations were performed. The complete blood count was 8,300/mm3 (neutrophils: 66.1%, lymphocytes: 33%, eosinophils: 2.8%) for the white cell count, 10.8 g/dL for the hemoglobin level, 32.2% hematocrit and 256,000/mm3 for the platelets count. The blood chemistry showed 79 mg/dL of fasting glucose, 15.4 mg/dL of urea nitrogen, 1.2 mg/dL of creatinine, 7.0 mg/dL of total protein, 3.6 mg/dL of albumin, 26 IU/L of AST, 35 IU/L of ALT, 357 IU/L of alkaline phosphatase, 0.5 mg/dL of total bilirubin and a normal prothrombin time. HBsAg and Anti-HCV were negative and anti-HBs was positive. The tumor marker study showed that AFP, CEA, CA 19-9 and CA 125 were all normal. A chest radiograph showed no abnormalities. Abdominal CT disclosed an about 3.6×4.8 cm sized ill-defined peripheral enhancing mass at segment 6 of the liver (Figure 1A) and another 3.3×2.6 cm mass of the same nature at the porta hepatis (Figure 1B). Ultrasonography-guided liver biopsy was done and biopsy of the mass at segment 6 of the liver disclosed an adenocarcinoma with moderate differentiation (Figure 2), and biopsy of the mass at the porta hepatis disclosed a mild lymphocytic infiltration. So, we search for the other primary origin; esophagogastrodaodenoscopy and colonoscopy were performed and they showed nonspecific findings. Breast ultrasonography showed multiple fibroadenomas in both breasts that were regarded as benign findings, category 3, and the bone scan showed no evidence of definite skeletal metastasis. We referred the patient to the department of general surgery for surgical intervention. Partial right lobectomy was performed. The surgical specimen of the liver mass disclosed the concurrent occurrence of hepatocellular carcinoma and cholangiocarcinoma, which can be called a "collision type carcinoma", and there were numerous calcified schistosome eggs and also fibrosis between the components of hepatocellular carcinoma and cholangiocarcinoma (Figure 3, 4). The lymph node specimen was positive for metastasis; it contained the HCC component. She was discharged with an improved condition. After one month from discharge, she was readmitted to the hospital due to her poor general condition. Abdominal CT disclosed new development of several small hypodense lesions at the remaining liver that suggested intrahepatic metastasis and new development of multiple metastatic lymphadenopathies along the celiac trunk and common hepatic artery, and there was about a 1cm sized lung nodule in left lower lobe, which was suspected to be hematogenous metastasis. The patient expired due to multiorgan failure on the 29th hospital day.
The occurrence of combined HCC-CC has been recognized for many years, but the histopathologic features of these tumors have rarely been detailed. Allen and Lisa7) classified combined HCC-CC into the following three categories; 1) separate tumors, each composed of only one type cell; 2) contiguous tumors, each of a different cell type that may mingle as they grow; and 3) individual lesions that have both types of cells and are interpreted to have arisen from the same site. Zachary and Goodman8) reported 24 case of this tumor, and three histologic types were encountered. Four cases were Type I or "collision tumors, and they were apparently the coincidental occurrence of both hepatocellular carcinoma and cholangiocarcinoma in the same patient. Twelve cases were Type II or "transitional tumors," in which there were areas of intermediate differentiation and an identifiable transition between HCC and CC. Eight cases were Type III or "fibrolamellar tumors" that resembled the fibrolamellar variant of HCC, but which also contained mucin-producing pseudoglands. In our case, the mass was categorized as a "collision tumor", which is the coincidental occurrence of both hepatocellular carcinoma and cholagiocarcinoma with no transitional area.
Several immunohistochemical studies have focused on the differential diagnosis of HCC and CC9-13). AFP is one of markers of HCC, but immunoreactivity for AFP is focal, if present at all13), and a significant number of cases are AFP negative. Similarly, CEA, which is a well-known marker of CC, is not always demonstrable. Thus, the usefulness of these antibodies for differentiation between HCC and CC is somewhat limited8, 14). Liver cells and biliary epithelial cells express cytokeratins 8 and 18, and biliary epithelial cells express 7, 8, 18 and 1910-12). Haratake et al. tested the usefulness of two antibodies, AE1 and Cam 5.2, and they reported that AE1 was the most useful marker for differentiating CC from HCC15). In our case, the CC areas were positive for cytokeratin 7 and 19, and they were weakly positive for Hepa-1 which is found in HCC. On other immnunohistochemical studies, Alpha-1-antitrypsin, IgG and carcinoembryonic antigen may be found in HCC, CC and in combined tumors, and so these antigens are of limited use in the differential diagnosis8).
There are differences in the clinical features between the histologic types of combined HCC-CC. In particular, Type III tumors differed from the other two types. The 4 patients with Type I tumors had an average age of 65 years, whereas the12 Type II tumors occurred in patients with an average age of 47 years, and the 8 Type III tumors occurred in patient with an average age of 30 years. Furthermore, five of the patients with Type III tumors were younger than 30 years. Although three of the four Type I tumors (75%) and eight of the ten Type II tumors (80%) occurred in cirrhotic livers, none of the Type III tumors occurred in the setting of cirrhosis. Similarly, there was a difference in survival after diagnosis. None of the patients with Type I or Type II tumors lived more than 2 months from the diagnosis. The eight patients with Type III tumors had an average survival of 14 months after diagnosis, and two patients lived longer than 2 years8). The survival time of our patient from the diagnosis was 2 months. In the report of the liver cancer study group of Japan, metastases of HCC were most frequent in the lung (45.1%), followed by lymph nodes (34.9%), and metastases of CC were most frequent in the lymph node (62.5%), followed by the peritoneum (46.3%). Lung metastasis of combined HCC-CC was the most frequent (83.3%), followed by metastasis to the peritoneum (66.6%) and the adrenal glands (50.0%)16). Yet in our case, the mass of the lymph node metastasis contained the HCC component.
In this case, the etiological relationship of schistosomiasis to combined HCC-CC is difficult to prove. It is possible that the HCC and CC each developed coincidentally or subsequently from fibrosis after recurrent inflammation at the site of schistosome infection.
In conclusion, we report here on a rare case with collision tumor, which was apparently the coincidental occurrence of both hepatocellular carcinoma and cholangiocarcinoma underlying schistosomiasis, and the lymph node metastasis contained the HCC component.
References
1. Ishak KG, Anthony PP, Sobin LH. Histologic typing of the liver 1994. 2nd ed. Berlin: Springer.
2. Tannapfel A, Bernicke M, Katalinic A, Uhlmann D, Kockerling F, Hauss J, Wittekind C. Frequency of p16(INK4A) alterations and K-ras mutations in intrahepatic cholangiocarcinoma of the liver. Gut 2000. 47:721–727PMID : 11034592.



3. Tannapfel A, Weinans L, Geissler F, Schutz A, Katalinic A, Kockerling F, Hauss J, Wittekind C. Mutations of p53 tumor suppressor gene, apoptosis, and proliferation intrahepatic cholangiocellular carcinoma of the liver. Dig Dis Sci 2000. 45:317–324PMID : 10711445.


4. Momoi H, Itoh T, Nozaki Y, Arima Y, Okabe H, Satoh S, Toda Y, Sakai E, Nakagawa K, Flemming P, Yamamoto M, Shimahara Y, Yamaoka Y, Fukumoto M. Microsatellite instability and alternative genetic pathway in intrahepatic cholangiocarcinoma. J Hepatol 2001. 35:235–244PMID : 11580146.


5. Momoi H, Okabe H, Kamikawa T, Satoh S, Ikai I, Yamamoto M, Nakagawara A, Shimahara Y, Yamaoka Y, Fukumoto M. Comprehensive allelotyping of human intrahepatic cholangiocarcinoma. Clin Cancer Res 2001. 7:2648–2655PMID : 11555575.

6. The Liver Cancer study Group of Japan. Survey and follow-up study of primary liver cancer in Japan: report 11. Acta Hepatol Jpn 1995. 36:208–218.

7. Allen RA, Lissa JR. Combined liver cell and bile duct carcinoma. Am J Pathol 1949. 25:647–655PMID : 18152860.


8. Goodman ZD, Ishak KG, Laugloss JM, Sesterhenn IA, Rabin L. Combined hepatocellular-cholangiocarcinoma: a histologic and immunohistochemical study. Cancer 1985. 55:124–135PMID : 2578078.


9. Ferrandez-Izquierdo A, Llombart-Bosch A. Immunohistochemical charaterization of 130 cases of primary hepatic carcinomas. Path Res Pract 1987. 182:783–791PMID : 2449680.


10. Johnson DE, Herndier BG, Medeiros LJ, Warnke RA, Rouse RV. The diagnostic utility of keratin profiles of hepatocellular carcinoma and cholangiocarcinoma. Am J Surg Pathol 1988. 12:187–197PMID : 2449824.


11. Fischer HP, Altmannsberger M, Weber K, Osborn M. Keratin polypeptides in malignant epithelial liver tumors: differential diagnosis and histogenetic aspects. Am J Pathol 1987. 127:530–537PMID : 2438941.


12. Balaton AJ, Nehama-Sibony M, Gotheil C, Callard P, Baviera EE. Distinction between hepatocellular carcinoma, cholangiocarcinoma, and metastatic carcinoma based on immunohistochemical staining for carcinoembryonic antigen and for cytokeratin 19 on paraffin sections. J Pathol 1988. 156:305–310PMID : 2465399.


13. Pasolero GC, Wakabayashi T, Oka T, Mori S. Tissue polypeptide antigen: a marker antigen differentiating cholangiolar tumors from other hepatic tumors. Am J Clin Pathol 1987. 87:168–173PMID : 3028121.


14. Harlimann J, Gardiol D. Immunohistochemistry in the differential diagnosis of liver carcinomas. Am J Surg Pathol 1991. 15:280–288PMID : 1847609.


Figure 1
Abdominal enhanced CT scan. (A) A 3.6×4.8 cm sized ill-defined enhancing mass is shown in segment 6 of the liver on the arterial phase (arrow). (B) Another 3.3×2.6 cm sized ill-defined enhancing mass is seen at the porta hepatis (arrowhead) and a part of the mass at segment 6 of the liver is seen during the portal phase (arrow).
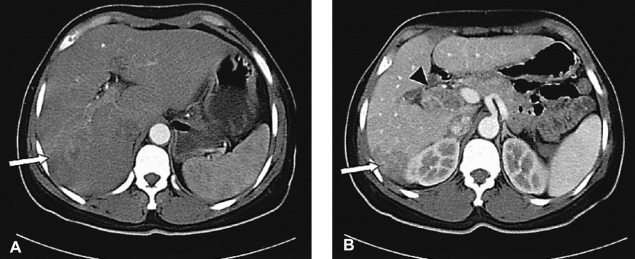
Figure 2
The microscopic findings of the mass in segment 6 show an adenocarcinoma with moderate differentiation by ultrasonography-guided needle biopsy (H&E stain, ×40).
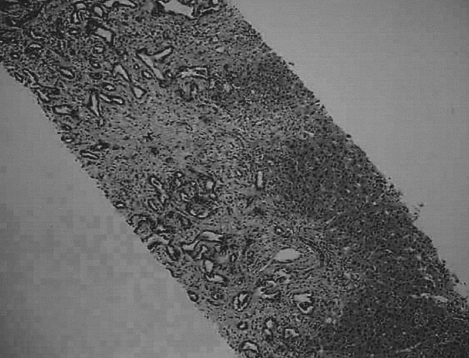
Figure 3
Microscopic findings. (A) Surgical specimen of the liver shows the histologically identified concurrent occurrence of hepatocellular carcinoma (left upper) and cholangiocarcinoma (right lower) (H&E stain, ×40). Several calcified schistosome eggs (arrowheads) with fibrosis are noted between the two components. (B) Hepatocellular carcinoma and (C) cholangiocarcinoma, respectively (H&E stain, ×100). (D) The lymph node biopsy was positive for metastasis, and the node contained hepatocellular carcinoma (H&E stain, ×400).



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print



