INTRODUCTION
Arteriovenous malformation (AVM) of the gastrointestinal (GI) tract is the single most common cause of significant GI bleeding when conventional diagnostic studies have failed to reveal a bleeding focus. In most of the cases reported, selective angiography was the only means by which a correct diagnosis could be made.1, 2) We report a young man with ventricular septal defect presenting as massive lower GI bleeding from a large colonic AVM involving a long segment of the left colon. The case was thought to belong to type 2 AVM according to Moore et al2). Type 2 AVM usually occurs in younger patients and is therefore considered a developmental anomaly. Unlike other reported type 2 cases, in most of which small bowels were usually involved1,2), the lesion was involving the left colon without involving the small bowel. Although the clinical significance is not clear, we could not find any AVM case, having ventricular septal defect, from the literature.
CASE REPORT
A 25-year-old Korean male was admitted due to 3 days′ history of hematochezia. Two weeks earlier, he had visited the outpatient clinic due to intermittent dull left lower quadrant pain and loose stool of 3 months′ duration. He had mild exertional dyspnea. Faint pansystolic murmur was audible along his left sternal border. A firm tender mass of 3–4 cm in size was palpable at his left lower abdomen. Colonoscopy revealed a small pedunculated polyp on the cecum and another small sessile polyp on the sigmoid colon The two polyps were removed with polypectomy. The mucosal folds were hypertrophic and the lumen was slightly narrow over a long segment of the sigmoid colon. Histologic examination of the removed polyps revealed lymphoid polyps. Mucosal biopsy from the narrow segment of the sigmoid colon disclosed only mild infiltration of chronic inflammatory cells.
On admission, his vital signs were stable. Hemoglobin was 11.0g/dl. Upper GI endoscopy was unremarkable. Colonoscopy revealed a small shallow ulceration at the previous polypectomy site on the cecum, and any evidence of recent bleeding could not be noted. Four days later, he experienced mild hematochezia again without any significant change in blood pressure and heart rate. Urgent sigmoidoscopy revealed fresh blood up to the splenic flexure, but the bleeding point was not clear.
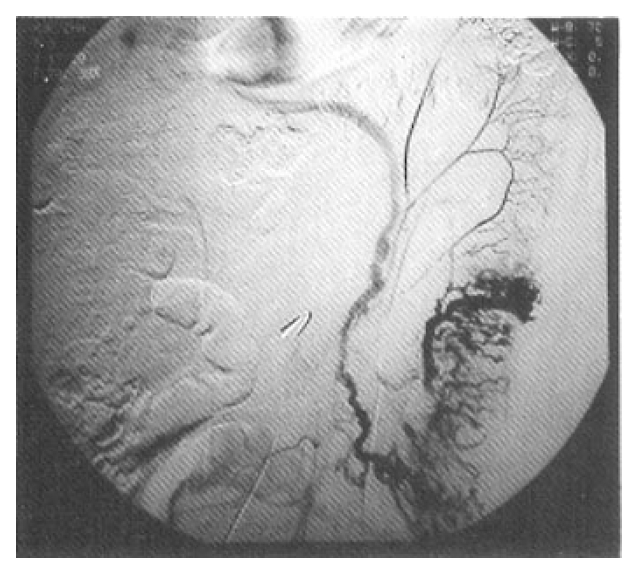
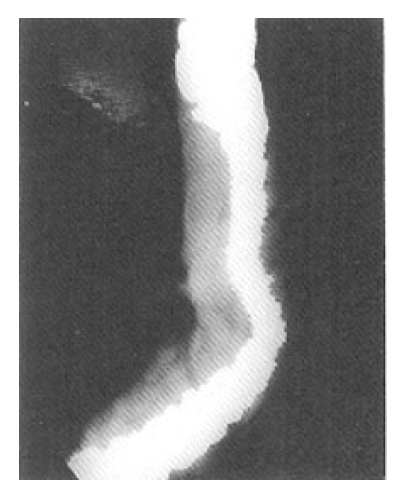
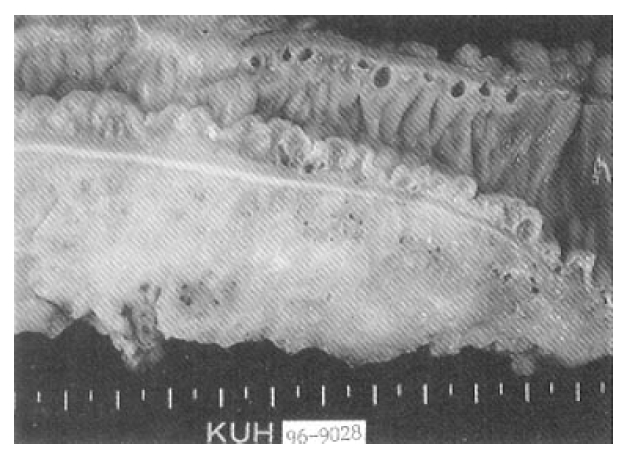
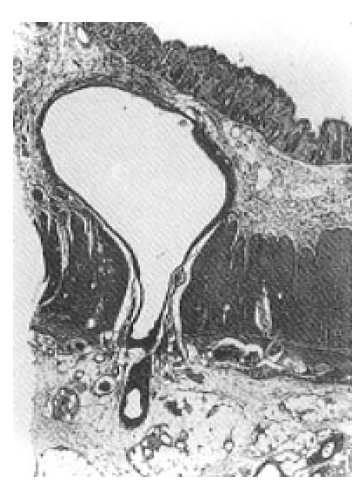
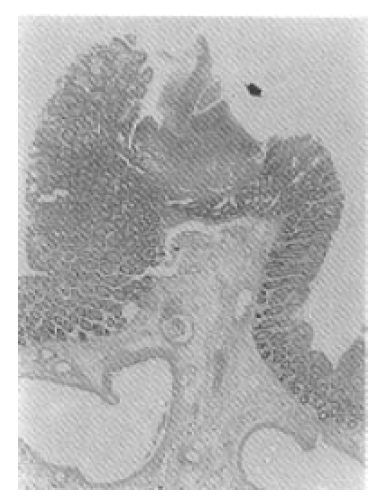
Selective inferior mesenteric arteriography demonstrated abnormal vasculature involving the sigmoid and distal descending colon without active bleeding (Fig. 1). Echocardiography and right cardiac catheterization demonstrated a ventricular septal defect with Qp/Qs ratio of 1.99. On the next day of the cardiac catheterization, he experienced a sudden massive lower GI bleeding and became hypotensive. On urgent laparotomy, a long abnormally hard thickened colonic segment with dilated tortuous vasculature involving the distal descending colon, sigmoid colon and upper rectum was found. Segmental colectomy, including the involved segment, was done. Post-resection barium angiography was done and the finding was similar to that of the preoperative angiography and confirmed the completeness of the resection. Barium enema was done with the resected specimen showing markedly thickened bowel wall (Fig. 2). The cut surface of the bowel wall demonstrated multiple ectatic blood vessels throughout the entire thickness of the wall (Fig. 3). Histology revealed abnormal ectaticvasculature (Fig. 4) and a bleeding point could be identified (Fig. 5).
His postoperative course was uneventful. A cardiac surgeon recommended open heart surgery for his ventricular septal defect, but he refused because he felt that his dyspnea was tolerable. He was discharged in a relatively good general condition.
DISCUSSION
Intestinal vascular malformation is one of the well known causes of recurrent GI bleeding3). The lesions are usually solitary, but may be multiple involving only the intestine or many organs and tissues throughout the body4).
The etiology of AVMs of the bowel is not known. A focus of the controversy has centered around whether AVM is a congenital or acquired disease. AVMs in younger patients might be congenital and those in older patients might be acquired.5) Intermittent bowel obstruction associated with vigorous peristalsis or frequent straining at stool may increase intraluminal pressure resulting in submucosal arteriovenous shunting.6) Partial obstruction of submucosal vein may lead to dilation and tortuosity of the venules and capillaries of the mucosa and also may lead to the loss of competency of the precapillary sphincter.3) Ultimately, an arteriovenous communication may appear.
Moore et al.2) classified AVMs of the GI tract into 3 types. Type 1 AVM is an angiodysplasia, an acquired degenerative lesion, occurring mainly in patients over the age of 60 years. Majority of its location is in the proximal ascending colon and cecum. The vascular ectasias are multiple and generally less than 5 mm in diameter. Type 2 AVM occurs in much younger patients and, therefore, considered to be a congenital vascular malformation. Histologically, these lesions are abnormal persistent communications between arteries and veins located primarily in the submucosa. There is arterialization of the veins with tortuosity, dilation, and thickening of the walls caused by smooth muscle hypertrophy and intimal thickening and sclerosis. The lesions are solitary or multiple, and they vary from a few millimeters in diameter to very large lesions with diffuse infiltration. Most of them are located in the small bowel. Type 3 AVM is a GI involvement of the hereditary hemorrhagic telangiectasia of Osler-Weber-Rendu type. Our case belongs to type 2 category, i.e. congenital AVM that is usually found in the younger age group.
A variety of reports have suggested that aortic stenosis may be found in about 10–60 % of patients with AVM, especially in the older population, i.e. type 1 according to Moore’s classification5,7,8). However, the apparent relationship between aortic stenosis and AVM remains unclear.5, 7) Our case had a ventricular septal defect, but its clinical importance as a possible association with AVM cannot be determined.
The major symptom of AVM is acute or chronic painless GI bleeding4). Other symptoms or complications are less common and include intussusception or obstruction due to a mass effect4).
Accurate identification of the bleeding sites may be technically difficult in the presence or absence of massive bleeding. High index of suspicion should be maintained for persons with chronic iron deficiency anemia or those with intermittent bleeding episodes undiagnosed by conventional tests or surgery. The most helpful preoperative method of diagnosis is selective arteriography.1,2,4,5) The importance of angiography cannot be overemphasized. Nearly all of the reported patients with proved AVMs had multiple barium contrast, roentgenographic and endoscopic examinations with negative results. Detection is usually impossible by conventional studies. The majority of AVMs are not grossly apparent even by inspection or palpation during exploratory laparotomy. Angiographic hallmarks of type 2 AVM are increased vascularity with pooling of contrast medium in dilated vascular channels, enlarged feeding arteries, early filling veins or vascular tufts, and slowiy emptying dilated tortuous intramural veins9). In our case, we could observe nearly all of the above arteriographic features. During angiography, all major arteries should be injected as the lesions may be multiple2, 4) Intraoperative endoscopy might be helpful for identifying the bleeding foci in some selected patients.
Treatment of AVM is surgical resection of the involved bowel segment, although endoscopic treatments were rarely reported as being effective to control bleeding.10,11) An immediate post-resection radiograph with barium injection into the arteries is recommended to assess the completeness of the resection.2, 4) AVM is so rare that it may be excluded from the list of differential diagnosis of patients in whom no definite source of GI bleeding can be identified by conventional diagnostic techniques.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





