INTRODUCTION
Nitric oxide (NO), produced by the conversion of the terminal guanidino nitrogen atoms from L-arginine, accounts for many of the biological properties of endothelium-derived relaxing factor (EDRF)1). NO synthesis is catalyzed by three distinct forms of nitric oxide synthases (NOS), i.e., brain (bNOS), inducible (iNOS) and endothelial constitutive (ecNOS) isozymes2). The importance of NO in the physiological control of blood pressure is now well established. It causes vascular relaxation by activation of soluble guanylate cyclase and, hence, increasing cyclic guanosine 3ŌĆ▓, 5ŌĆ▓-monophosphate (cGMP) levels in the smooth muscle3). Therefore, an alteration in NO synthesis may be a significant factor in the pathogenesis of hypertension. A decreased responsiveness to endothelium-dependent vasodilators is characteristically seen in isolated arteries from various models of experimental hypertension4ŌĆō7).
A large number of studies evidence the capacity of the kidney to produce NO and its relevant role in renal function8ŌĆō12). It has been demonstrated that the kidney is very sensitive to the reduction of NO, as low doses of NOS inhibitors reduce sodium and water excretion without affecting renal hemodynamics or systemic arterial pressure8). The kidney has an intrinsic mechanism, in which an increased renal perfusion pressure promotes sodium excretion, a phenomenon called pressure natriuresis13). It has been shown that chronic inhibition of NO synthesis impairs sodium excretion, resulting in right shift of the pressure-natriuresis curve and hypertension14). In spontaneously hypertensive rats (SHR), pressure-natriuresis is also blunted15) and papillary blood flow is reduced16). Recently, Larson et al.17) have shown that administration of L-arginine to SHR restores the pressure-dependent increase in renal medullary hemodynamics in association with restoration of pressure-natriuresis. These results suggest that a defective NO pathway may be involved in the blunted pressure-natriuresis. However, the precise status and role of the NO-cGMP pathway in the kidney in SHR is not clear.
The present study was aimed at investigating whether the hypertension is related with an altered activity of NO system in the kidney of SHR. The expression of NOS isozymes and tissue NO levels were determined in the kidney isolated from SHR and their normotensive control Wistar-Kyoto rats (WKY) by Western blot analysis.
MATERIALS AND METHODS
1. Animals
Experiments were carried out using adult (12-week old) male SHR and WKY. Systolic blood pressure was measured in a conscious state by the tail-cuff method. Rats were killed by decapitation. Blood was collected from the trunk, and centrifuged (3,000 rpm for 30 min) for the determination of plasma NO contents. Kidneys were rapidly removed, immediately frozen in liquid nitrogen and stored at ŌłÆ80┬░C until extraction.
2. Colorimetric Assay of Nitrite/Nitrate
NOx (nitrite/nitrate) contents in the kidney and plasma were measured with a colorimetric nitric oxide assay kit (Oxford). A microplate was used to perform enzyme reactions in vitro. For spectrophotometric assay of nitrite with Griess reagent, 80 ml MOPS (50 mmol/L)/EDTA (1 mmol/L) buffer and 5 ╬╝l tissue samples were added to wells. Nitrate reductase (0.01 U) and 10 ╬╝l NADH (2 mmol/L) were added to the reaction mixture, and the plate was shaken for 20 minutes at room temperature. Color reagents, sulfanilamide and N-(1-Naphthayl) ehylenediamine dihydrochloride were added, and absorbance values at 540 nm were read in a microtiter plate reader (Bio-Rad model 3550). The concentration of nitrite/nitrate was estimated from a standard curve, which was constructed with the use of standard reagents included in the assay kit.
3. Protein preparation
The cortex, outer medulla and inner medulla from frozen kidney tissues were dissected and were homogenized with Polytron homogenizer at 3,000 rpm in a solution containing 250 mmol/L sucrose, 1 mmol/L EDTA, 0.1 mmol/L phenylmethylsulfonyl fluoride and 50 mmol/L potassium phosphate buffer at pH 7.6. Large tissue debris and nuclear fragments were removed by two consecutive low speed centrifuge spins (3,000g, 5 min; 10,000g, 10 min). The protein concentration of the homogenate was determined by the method of Bradford18), with bovine serum albumin as a standard. In the case of cortex, the membrane-bound protein was further centrifuged at 100,000g for 60 min. The pellet was resuspended for protein blotting of ecNOS and the supernatant was used for blotting of bNOS and iNOS.
4. Western blot analysis
Protein samples were electrophoretically size-separated with a discontinuous system consisting of a 7.5% polyacrylamide resolving gel and 5% polyacrylamide stacking gel. High-range molecular weight markers (Biorad; Hercules, CA, USA) were loaded as size standard. An equivalent amount of total tissue protein (100 ╬╝g) was loaded on each lane. After separation, the proteins were electrophoretically transferred to a nitrocellulose membrane at 20 V overnight. The membranes were washed in Tris-based saline buffer (pH 7.4) containing 1% Tween-20 (TBST), blocked with 5% nonfat milk in TBST for one hour and incubated with a 1:2,000 dilution of monoclonal mouse anti-bNOS, anti-ecNOS and anti-iNOS antibodies (Transduction Laboratories; Lexington, KY, USA) in 2% nonfat milk/TBST for one hour at room temperature. The membranes were then incubated with a horseradish peroxidase-labeled goat anti-mouse IgG (1:1,000) or goat anti-rabbit IgG in 2% nonfat milk in TBST for 2 hours. The bound antibody was detected by enhanced chemiluminescence on X-ray film or hyperfilm (Amersham, Little Chalfont, Buckinghamshire, England). The membranes were stripped between incubations with different antibodies in a Tris-buffered solution containing 2% sodium dodecyl sulfate and 100 mmol/L ╬▓-mercaptoethanol at 50┬░C.
RESULTS
2. NOx contents and NOS expression in the kidney
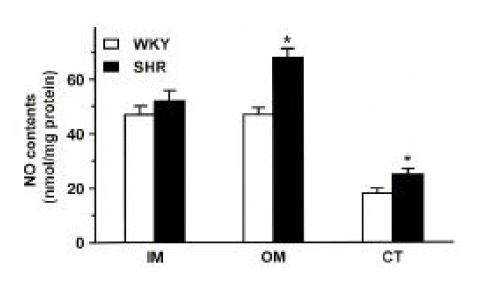
Fig. 2 shows NOx contents in the inner medulla, outer medulla and cortex of the kidney in SHR and WKY. The NOx contents were higher in the outer medulla and cortex of the kidney by about 50% and 60%, respectively, in SHR compared with those in WKY.
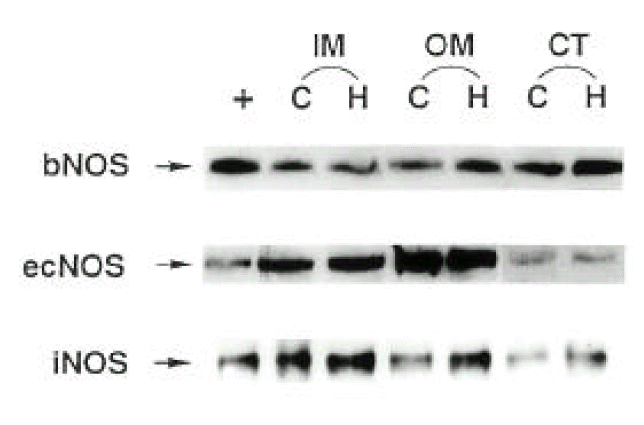
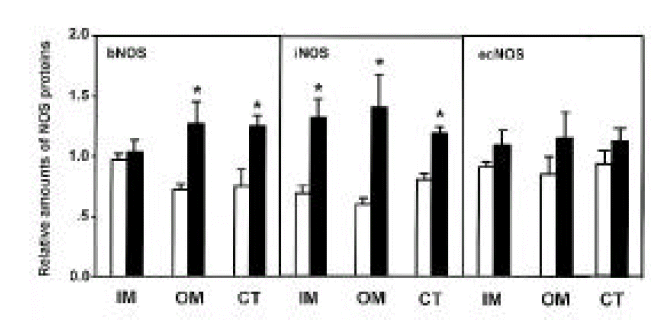
Three isoforms of NOS (bNOS, iNOS, ecNOS) were determined in the renal cortex and outer and inner medulla of SHR and WKY by Western blot analysis (Fig. 3). Anti-bNOS, anti-iNOS and anti-ecNOS antibodies recognized protein bands with molecular sizes of 155, 130 and 140 kDa, respectively. Fig. 4 shows densitometric analysis of NOS in the kidney. ecNOS protein expression did not significantly differ between SHR and WKY. However, bNOS proteins were expressed higher In the outer medulla and cortex by 80% and 70%, respectively, and iNOS proteins were higher in the inner medulla, outer medulla and cortex by 95%, 135% and 50%, respectively, in SHR.
DISCUSSION
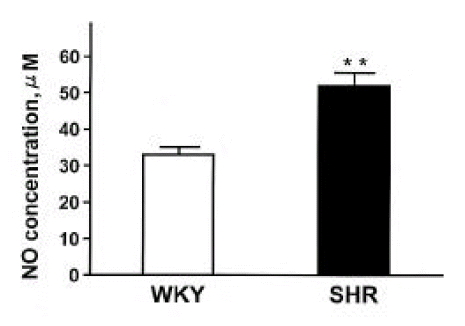
It is well known that acetylcholine-induced vasodilation is impaired in SHR19ŌĆō21), however, of which precise mechanisms are not fully understood. A growing number of studies22ŌĆō25) have demonstrated that NO synthesis may be increased in SHR. It was reported that an inhibition of NOS produced a greater reduction of vasorelaxation to acetylcholine26) and an exaggerated hypertension in SHR27). Furthermore, the present study showed that plasma NOx (nitrite/nitrate) concentration and NOx contents in the kidney were significantly higher in SHR compared with those in WKY. It has been known that the release of NO by endothelial cells can be altered by changes in blood flow28), and their mRNA and protein for ecNOS can be induced by mechanical forces29). The augmented expression of ecNOS may play an important role in the compensatory mechanisms to counteract the hypertension.
The present study showed that renal NOx contents and NOS protein expression in the medulla were higher than in the cortex. These findings are consistent with previous studies revealing the largest signal for constitutive NOS protein expression in the inner medullary collecting duct30), and with the report showing the highest amount of cGMP in the inner medulla, when stimulated with acetylcholine31). These results suggest that the medullary NO system plays a more important role in renal function, such as control of sodium and water excretion.
Tubuloglomerular feedback (TGF) is the critical component of a negative feedback control system that effectively buffers the hemodynamic influences on GFR and stabilizes the amount of NaCl entering the distal nephron32). In the kidney, bNOS protein has been demonstrated exclusively in the macula densa cells, and NO produced in these cells counteract the TGF-mediated vasoconstriction33, 34). This finding indicates that, under normal conditions, NO is one of major determinants of TGF sensitivity. SHR are known to have a sensitive TGF response during development of hypertension35, 36). The exaggerated response could contribute to a lower glomerular filtration and, perhaps, to the development of hypertension37). Thorup and Persson38) compared the effects of intratubular inhibition of NO by [NŽē-nitro-L-arginine (L-NNA)] on the TGF between SHR and WKY rats, and showed that L-NNA did not change either sensitivity or the magnitude of the TGF response in SHR, whereas their normotensive control strains responded with a very strong resetting toward higher sensitivity. This finding indicates that NO-mediated vasodilation that counteracts TGF-induced vasoconstriction of the afferent arteriole is less pronounced in SHR than in their normotensive controls. Our study demonstrated that the expression of bNOS protein in renal cortex and outer medulla in SHR was higher than in WKY. Since bNOS in macula densa seems to attenuate the afferent arteriolar constriction induced by the TGF33, 34), enhanced bNOS expression would be expected to increase GFR and sodium excretion which act as a compensatory function opposing the hypertension.
Pressure-natriuresis is associated with significant increase in vasa recta blood flow39). It has been postulated that the mechanism, through which changes in medullary hemodynamics result in pressure-dependent increase in urinary sodium excretion, is due to transmission of pressure from the systemic circulation to the renal interstitium through the renal microcirculation40). In SHR, papillary blood flow in response to increased renal perfusion pressure is reduced, and pressure-natriuresis is blunted41). Hence, the attenuated response of renal medullary hemodynamics to change in renal perfusion pressure has been postulated to be causally linked to the abnormal pressure natriuresis in the SHR41, 42). The restoration of the vasa recta hemodynamic response with L-arginine in SHR supports a role for NO in the regulation of medullary blood flow17). Ikenaga et al43) reported that intravenous administration of L-arginine increase urinary excretion of NO metabolites in normotensive animals, and in SHR to a similiar degree, suggesting that, in the SHR, the ability to synthesize NO is preserved but the renal response to NO is impaired. Our study also demonstrated that the basal production of NO was higher in the kidney in SHR compared with that in WKY. bNOS proteins were expressed higher in the outer medulla and cortex, and iNOS proteins were higher in the inner medulla, outer medulla and cortex in SHR. These results suggest that impaired effect of NO or activation of other vasoconstricting factors may be responsible for the blunted pressure natriuresis in SHR rather than defective NO production.
In conclusion, the NO generation may not be impaired, but rather increased in the kidney of SHR. It is likely that an altered expression of NOS isozymes plays a counter-regulatory role secondary to the hypertension in SHR.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





