INTRODUCTION
Type 2 DM is caused mainly by insulin secretary defect and peripheral insulin resistance but the relative importance of these two factors is a matter of debate. The basal proinsulin level is more increased in type 2 DM than in normal control and hyperproinsulinemia is thought to be a predictor of subsequent development of type 2 DM1).
Proinsulin constitutes 2ŌĆō6% of the immunoreactive insulin (IRI)-like material secreted by ╬▓-cells of the pancreas under normal basal conditions, but appears for approximately 10ŌĆō20% of the circulating total IRI because it is cleared from the plasma more slowly than insulin2, 3). A disproportionate elevation of proinsulin levels has been described in patients with type 2 DM and has been attributed to increased demand for insulin in diabetic patients with ╬▓-cell dysfunction, leading to depletion of mature insulin-rich granules and subsequent mobilization of immature secretary granules rich in proinsulin (PI)4, 5). Furthermore, elevated proinsulin concentrations have been demonstrated in non-diabetic twins of patients with type 1 DM and were interpreted as an indication of early subclinical ╬▓-cell dysfunction6).
However, there are some conflicting reports on the relationship between insulin resistance and proinsulin/insulin ratio. Wang et al reported that the fasting proinsulin to insulin ratio had no relationship to the degree of insulin res istance although proinsulin and insulin concentrations were increased in insulinresistant, nondiabetic subjects7). On the other hand, Haffner and associates published evidence that the ratio of fasting proinsulin to insulin concentration was increased in individuals diagnosed as having the insulin resistance syndrome8).
In this study, insulin and proinsulin levels were measured in eighty-six type 2 diabetic patients and seventy healthy control subjects in order to investigate the difference of insulin, proinsulin and proinsulin/insulin ratio in these two groups. Secondly, we wanted to know how the serum insulin, proinsulin and proinsulin/insulin ratio were correlated with the markers of insulin resistance and ╬▓-cell function.
MATERIALS AND METHODS
1. Materials
Eighty-six type 2 diabetic patients were selected according to NDDG/WHO criteria from patients attending Korea University Hospital from 1997 to 1998. Age, sex, duration of diabetes, diabetic medication, body mass index (BMI) and laboratory examinations were recorded (see below). After the subjects had been resting in a sitting position for at least 5 minutes, their blood pressure was measured with an appropriate-sized cuff on the right upper arm. Blood pressure was measured two times, and the average of the two measurements was used throughout the study. The subjects were cons idered to have hypertension if they had a blood pressure of greater than 140 mmHg in systole or 90 mmHg in diastole or if they were receiving antihypertensive medication. These hypertensive patients were treated by an angiotensin enzyme inhibitor, a calcium channel blocker or an alpha blocker. Cardiovascular disease was proven by electrocardiogram, echocardiogram or coronary angiogram. Seventy healthy non-diabetic subjects of comparable age and sex distribubution acted as a control group.
2. Methods
Venous blood was taken from all subjects after overnight fasting and the serum was stored at ŌłÆ20┬░C. Specific insulin concentration was determined by radioimmunoassay (RIA) using a human-specific antibody (human insulin specific RIA kit, Linco Research, St Charles, MO, U.S.A.). This antibody selectively measures human insulin with practically no crossreactivity (<0.2%) to proinsulin or primary circulating split form, des 31,32 proinsulin. Proinsulin was also measured by a RIA kit (Linco Research, USA). This kit measures total proinsulin in serum or plasma, and the cross-reactivity is less than 0.1% to insulin and C-peptide. Insulin was defined as the sum of specific insulin and proinsulin. Total serum cholesterol concentration was measured through the reaction of cholesterol oxidase-peroxidase, and total serum triglyceride concentration was measured by glycerol-1-phosphate dehydrogenase-diaphorase using CX7 (Beckman Instruments, Bera, CA, USA). HDL cholesterol was measured by magnesium precipitation method.
We used StudentŌĆÖs unpaired t-tests for comparison of quantitative variables and used chi-square tests for comparison of proportions. PearsonŌĆÖs correlation coefficients and multiple regression analysis were also performed. A significance level of 5% was chosen for all the tests (p value=0.05). Statistical analyses were performed with SPSS-win software for IBM computers C Spss Inc.
RESULTS
1. Features of type 2 diabetic patients and control subjects (Table 1)
Table 1 shows the clinical and biochemical characteristics of the type 2 diabetic patients and the control subjects. Typically the diabetic patients had higher fasting blood glucose. Age, sex ratio, BMI, triglyceride, total cholesterol and HDL cholesterol were similar in both groups. The mean duration of diabetes was 7.5┬▒6 years and HbA1C was 7.9┬▒ 1.5% in diabetic patients.
2. Serum insulin, proinsulin and insulin/proinsulin ratio
Insulin level was higher in diabetic patients (122.4┬▒70.4 vs. 82.5┬▒22.8 pmol/L, p<0.001). Proinsulin level was also higher in diabetic patients than control subjects (21.1┬▒13.5 vs. 7.9┬▒2.9 pmol/L, p<0.001). Proinsulin/insulin ratio was significantly higher in type 2 DM than control (0.22┬▒0.10 vs. 0.097┬▒0.03, p<0.001) (Table 1).
When the diabetic patients were divided by treatment modality, ten patients had been treated with diet only and seventy-six patients with oral hypoglycemic agents. Insulin and proinsulin levels were similar in both diet only group and oral hypoglycemic group (insulin: 141.7┬▒84.6 pmol/L vs. 120.5┬▒68.9 pmol/L, p>0.05, proinsulin 20.1┬▒16 pmol/L vs. 23.1┬▒13.3 pmol/L, p>0.05). On the contrary, proinsulin/insulin ratio was significantly elevated in oral hypoglycemic agent group than diet only group (0.18┬▒0.06 vs. 0.13┬▒0.04, p=0.035).
3. Correlation between the insulin, proinsulin and proinsulin/insulin ratio and other metabolic parameters in diabetic patients
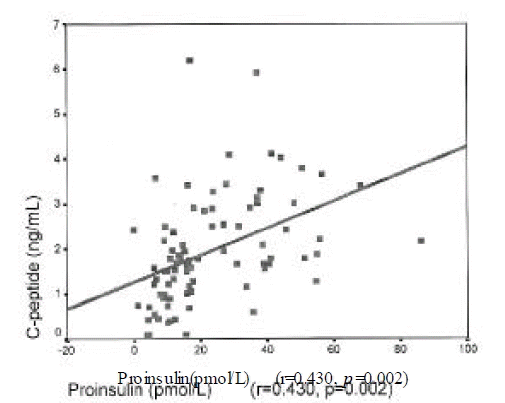
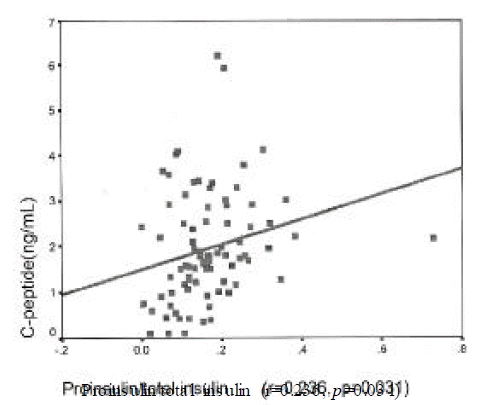
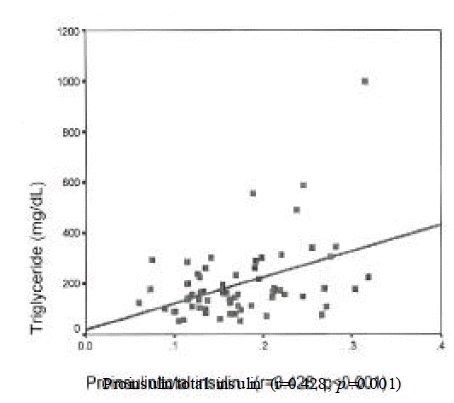
Insulin level was correlated with urinary albumin excretion rates(r=0.224, p=0.025) and body mass index(r=0.269, p=0.014). Proinsulin level was correlated with fasting C-peptide(r=0.43, p=0.002), postprandial 2 hour blood glucose(r=0.213, p=0.05) and triglyceride (r=0.28, p=0.022). Proinsulin/insulin ratio was positively correlated with fasting C-peptide(r=0.236, p=0.031), fasting blood glucose(r=0.264, p=0.015), postprandial 2 hour blood glucose(r=0.277, p=0.001) and triglyceride (r=0.428, p<0.001)(Figure 1ŌĆō4).
In multiple regression analysis, insulin level was correlated with urinary albumin excretion rate and triglyceride. Proinsulin level was correlated with fasting C-peptide, triglyceride, total cholesterol and LDL cholesterol. Proinsulin/insulin ratio was correlated with fasting C-peptide and postprandial 2 hour blood glucose.
4. Correlation between the insulin, proinsulin and proinsulin/insulin ratio and other metabolic parameters in control subjects
In control subjects, insulin concentration was correlated with triglyceride(r=0.366, p=0.002). Proinsulin/insulin ratio was correlated with age(r=0.241, p=0.044).
In multiple regression analysis, insulin level was correlated with BMI, systolic BP and triglyceride. Proinsulin level was correlated with age, BMI, triglyceride, waist/hip ratio and systolic blood pressure. Proinsulin/insulin ratio was positively correlated with age, BMI, waist/hip ratio and systolic blood pressure.
DISCUSSION
In our study, insulin, proinsulin and proinsulin/insulin ratio were significantly elevated in type 2 DM patients than in healthy control subjects. In both diabetic and control subjects, insulin and proinsulin levels were correlated with fasting c-peptide, triglyceride, total cholesterol, LDL cholesterol and urinary albumin excretion rates which are components of insulin resistance syndrome, whereas proinsulin/insulin ratio was positively correlated with fasting c-peptide and postprandial 2 hour blood glucose in type 2 DM patients. Relative increase of proinsulin in response to ingested glucose seemed to be related to the beta cell secretary defect. Also, proinsulin/insulin ratio was increased with age in control subjects, which supported the theory of age-related decline in ╬▓-cell function and increased risk of type 2 DM.
Proinsulin is converted to insulin in beta cell granules. Conversion involves endoproteolytic cleavage to equimolar insulin and C-peptide9). Under normal circumstances, proinsulin conversion is largely completed before secretion and neither the intact prohormone nor conversion intermediates are thus encountered in large quantities in the circulation. In some pathological conditions, including type 2 DM, unusually high ratio of proinsulin to insulin has been reported 10, 11). Even under relatively mild hyperglycemic circumstances, the proportion of circulating proinsulin rises from 10% normally to 15ŌĆō22%. As hyperglycemia worsens, the relative contribution of proinsulin in the circulation can rise to more than 40%12). Saad et al. showed that fasting proinsulin levels are related to blood glucose in type 2 DM13) and Mykk├żnen et al. reported that serum proinsulin levels are increased even in the impaired glucose tolerant state14). Several recent studies have suggested that increased fasting proinsulin concentration and the ratio of fasting proinsulin/insulin could predict the development of type 2 DM in normal subjects1, 14). Increased proinsulin concentrations are thought to be an early indicator of failing pancreas. The cause of hyperproinsulinemia may be due be a pancreatic ╬▓-cell defect that is augmented by the increased demand placed on the ╬▓-cell by hyperglycemia4, 5, 10ŌĆō12) rather than a decreased clearance of proinsulin-like molecules from the circulation.
Radioimmunoassay (RIA) is the most commonly used approach for measuring insulin-like products in plasma. However, measurement of immunoreactive insulin (IRI) by this method has been complicated by the fact that most RIAs tend to be nonspecific in that they do not discriminate between insulin and its precursors15). This lack of specificity means that, in most RIAs, estimates of IRI include intact proinsulin and its conversion intermediates, 32,33- and 65,66-split proinsulins, as well as des-31,32-and des-64,65-split proinsulins. Yet, in this study, specific insulin concentration was determined by means of highly specific two-sites monoclonal-antibody-based immunoradiometric assays. This antibody selectively measures human insulin with practically no crossreactivity (<0.2%) to proinsulin or the primary circulating split form, des-31,32-proinsulin. Proinsulin was also measured by the same method.
Although proinsulin/insulin ratio would reflect insulin secretory defects, the relationship between the Proinsulin/insulin ratio and insulin resistance is controversial. Wang et al. demonstrated that fasting proinsulin and insulin concentrations were increased in insulin-resistant, non-diabetic subjects but the ratio of proinsulin/insulin did not vary as a function of insulin resistance7). On the other hand, Haffner and associates published evidence that the ratio of fasting proinsuln/insulin concentration was increased in individuals diagnosed as having the insulin resistance syndrome8). One obvious difference was that their study population was quite different. HaffnerŌĆÖs subjects were heavier (BMI, 30 kg/m2), predominantly Mexican-American, and approximately 25% of their population had either IGT or hypertension. In this study, even though total insulin and proinsulin were correlated with several components of insulin resistance syndrome, the Proinsulin/insulin ratio was positively correlated with fasting c-peptide and postprandial 2 hour blood glucose. This disproportionately elevated proinsulin concentration after 2 hours of glucose ingestion could also be due to hypersecretion of immature proinsulin-rich granules in beta cells in response to an increased demand for insulin. Therefore, the proinsulin/insulin ratio would reflect insulin secretory defect rather than insulin resistance in our study.
Recently, it has been suggested that hyperinsulinemia may be a common element accounting for the association of obesity, type 2 DM, dyslipidemia and hypertension16ŌĆō18). These metabolic abnormalities are unquestioned risk factors for cardiovascular disease. Nagi et al. examined the relationship among levels of proinsulin, lipids and plasminogen activator inhibitor type 1 (PAI-1) in 51 subjects with type 2 DM19). They found that plasma proinsulin level in these type 2 DM subjects was correlated positively with total cholesterol, triglycerides and PAI-1 and negatively with HDL cholesterol. The relationship of proinsulin level to cardiovascular risk factors was recently examined by Haffner et al. in a cohort of non-diabetic subjects from the San Antonio Heart Study20). In these 260 non-diabetic subjects, fasting proinsulin level was positively correlated with body adiposity and triglycerides and negatively with HDL cholesterol. Also in this study, proinsulin level is correlated with many components of insulin resistance syndrome; fasting C-peptide, triglyceride, total cholesterol and LDL cholesterol. These results support the theory that proinsulin would be an element of insulin resistance syndrome. Furthermore, Schneider et al. indicated that proinsulin increased endothelial cell expression of plasminogen activator inhibitor type-1 (PAI-1) mRNA with marked increase in PAI-1 activity, potentially contributing to vasculopathy in patients with type 2 DM21).
The effect of sulfonylurea on the secretion of proinsulin and proinsulin/insulin ratio is not well known. Kahn et al. reported that, in the twenty-two type 2 DM subjects receiving sulfonylurea, the Proinsulin/insulin ratio was greater in the group of subjects taking sulfonylurea22). Those subjects tended to have higher proinsulin level, whereas true insulin level was not different between the two groups. Prigeon et al. demonstrated that this difference in the Proinsulin/insulin ratio was not probably due to the effect of these agents to alter the ratio, as they found that sulfonylurea withdrawal did not change Proinsulin/insulin ratio23). Subjects taking sulfonylurea would be expected to have poorer ╬▓-cell function. In this study, the ratio of proinsulin/insulin is higher in type 2 DM patients receiving sulfonylurea than diet only group. Neither the increased proinsulin level nor the decreased insulin level is responsible for the increased proinsulin/insulin ratio. But there is a tendency of higher proinsulin and lower insulin level in sulfonyurea-treated group. Those subjects receiving sulfonylurea have longer diabetes duration, higher fasting and postprandial 2 hour blood glucose, and they are relative insulin deficiency state than diet only subjects. Proinsulin/insulin ratio may reflect these differences between sulfonylurea-treated group and diet only group. Nevertheless, it is difficult to assess the effect of sulfonylurea on the proinsulin/insulin ratio in our study because the number of these groups are very different.
In conclusion, both insulin deficiency and insulin resistance are involved in the development of type 2 DM and these factors can contribute to the changes of circulating insulin, proinsulin levels and Proinsulin/insulin ratio. In our study, the serum levels of insulin and proinsulin seem to be associated with several markers of insulin resistance. However, the Proinsulin/insulin ratio might represent beta cell function rather than insulin resistance. It also increases with age, which supports the theory of age-related decline in ╬▓-cell function and increased risk of type 2 DM. Proinsulin/insulin ratio would be different between many ethnic groups according to their insulin secretory defect and insulin resistance status. In Korean people, proinsulin level would be increased early even in the glucose intolerant state because they have relative insulin secretory defect than Caucacians. More studies about the mechanisms of elevated proinsulin and proinsulin/insulin ratio are needed to clarify the pathophysiology of type 2 DM, especially in our country.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





