INTRODUCTION
Actinomycosis is an indolent infectious disease characterized by pyogenic response and necrosis, followed by intense fibrosis1). Several syndromes have been associated with Actinomyces infection, including cervicofacial, pulmonary, abdominal, female genital and disseminated actinomycosis. Clinical presentations of thoracic actinomycosis have changed considerably over the last decades with the reduction of its incidence. The presenting signs of actinomycosis are unresolved pneumonia or pulmonary infiltrate or a mass on routine chest radiograph2). The infection typically spreads without regard to anatomic barriers, but involvement of the major bronchi is exceptionally uncommon3,4).
CASE
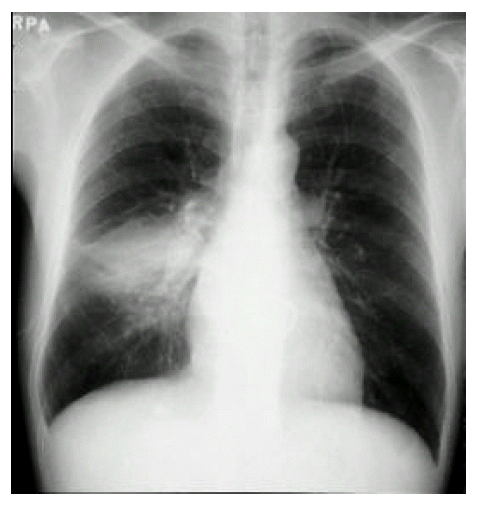
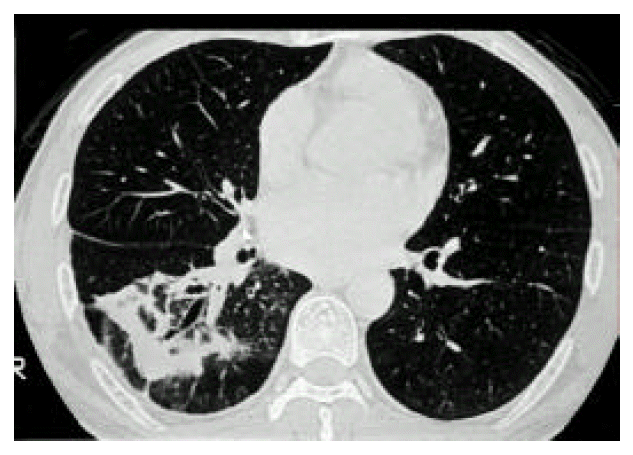
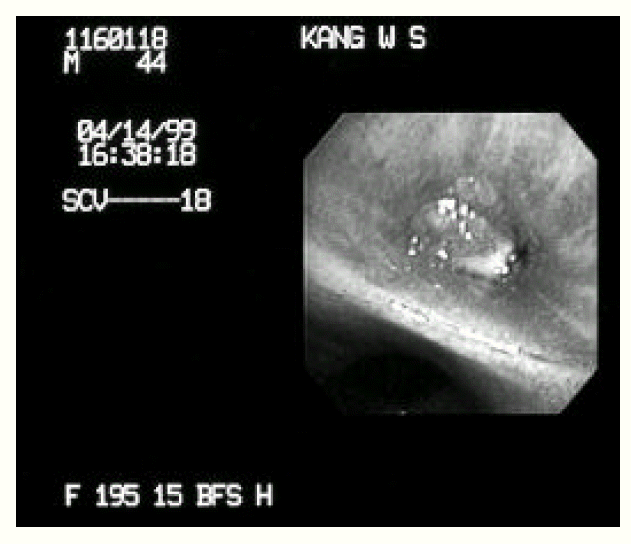
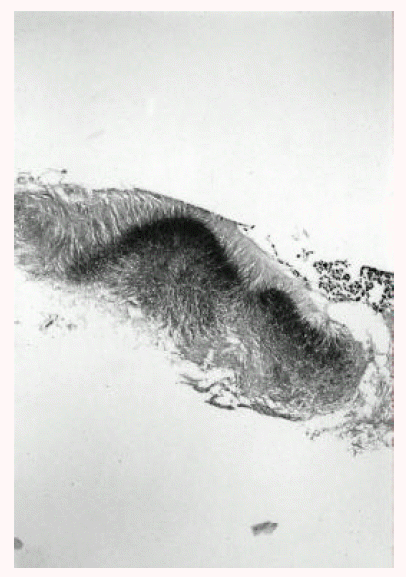
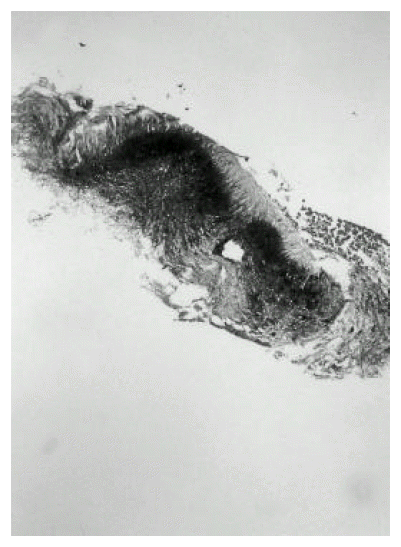
A 43-year-old man was admitted to our hospital with one-month history of productive cough. He had a 3-year history of mitral stenosis and an operation for mitral valve replacement. There was no history of recurrent infection or other evidence of immunodeficiency. Physical examination revealed a temperature of 36.8┬░C. Chest examination revealed an inspiratory crackle in the right lower lung field. Laboratory tests revealed a leukocyte count of 11,600/╬╝L and hemoglobin of 13.2 g/dL. Differential cell count disclosed the following values: neutrophils 76%; lymphocytes 17%; monocytes 5%; eosinophils 1.7%; basophils 0.3%. Chest radiograph showed focal consolidation of the right lower lobe (Figure 1). Computed tomography of the chest demonstrated patchy air-space consolidation and atelectasis of the right lower lobe (Figure 2). Gram stain showed many Gram positive cocci; AFB smear and culture for Mycobacterium tuberculosis were negative; there were no fungi. Fiberoptic bronchoscopy showed subtotal occlusion of the right superior segment of the lower lobe by a yellow-white, stony-hard mass, which was surrounded by inflamed and edematous bronchial mucosa (Figure 3). Histologically, the biopsy specimen revealed an inflammatory cellullar infiltration and colonies which were formed of a radiating network of filaments staining intensely with hematoxylin (Figure 4). The Gram stain also showed a thin, filamentous, Gram-positive, branching organism. (Figure 5). The patient was initially treated with 10 million units of intravenous penicillin daily for two weeks, followed by oral amoxacillin/clavulanic acid. After 3 months of therapy, the clinical manifestations and radiologic findings were markedly improved (Figure 6).
DISCUSSION
Actinomycosis is a chronic suppurative infection which infiltrates mucosa-associated tissues. Species of Actinomyces are gram-positive, anaerobic microorganisms belonging to the resident flora of the oropharynx and gastrointestinal tract and are found in 30ŌĆō50% of normal saliva specimens1). The term Actinomycete, which is of Greek origin, means ray fungus; however, actinomycosis is due to a gram-positive anaerobic bacterium. Bollinger described the micro-organism in 1877 as the causative agent of lumpy jaw in cattle and named it Actinomyces bovis5). In 1891, Wolf and Israel found a similar micro-organism from pulmonary abscess and named it Actinomyces israelii6). Although most cases of human actinomycosis are due to A. israelii, less common agents are Actinomyces naeslundii, Actinomyces viscosus, Actinomyces odontolyticus, Actinomyces meyeri and Propionibacterium propionicum. The disease is classically divided into three types, depending on the anatomic sites involved; cervicofacial, abdominopelvic and thoracic. Cervicofacial infection is the most frequent manifestation, with thoracic actinomycos is accounting for approximately 20% of cases7). Abdominal and pelvic manifestations are less frequently observed. Aspiration of organisms from the oropharnx is the usual source of thoracic Actinomyces infection. Common primary lesions in the lung involve the peribronchial tissues, bronchioles and alveoli. Direct extension may occur from disease in either the head and neck or abdominal cavity. The organisms may then spread from the lung to the pleura, mediastinum and chest wall without regard for normal anatomic barriers. The reason for this is unclear but may relate to the proteolytic activity of the bacteria.
Infection may occur in individuals of all ages. The peak incidence is reported to be in the mid-decades, with cases in individuals younger than 10 and older 60 years being less frequent8). Nearly all series have reported males to be infected more frequently than females, at an approximately 3:1 ratio9). Plausible but unproven explanations for this discordance include poorer dental hygiene and increased oral trauma in males. Studies of the occurrence of actinomycosis estimated a yearly incidence of 1:100,000 in the Netherlands and Germany in the 1960s and 1:300,000 in the Cleveland area during the 1970s, making this disease uncommon but not rare8). A pivotal step in the pathogenesis of actinomycosis is disruption of the mucosal barrier.
Oral and cervicofacial diseases are frequently associated with dental procedures, trauma and oral surgery. The typical lesion consists of abscesses filled with neutrophils and surrounded by dense fibrous tissue. Macrophages, plasma cells and lymphocytes are abundant in the periphery of lesions. Giant cells are uncommon and epithelioid granuloma are exceedingly rare. In tissue, Actinomyces organisms tend to grow in dense microcolonies or granules that may reach 4mm in size. These are often called sulfur granules because they are usually yellow, although they do not contain much sulfur. The bacteria are embedded in mucopolysaccharide matrix containing substantial amounts of Ca3 (PO4)27). The hallmark of actinomycosis is the formation of the yellow sulfur granules. The micro-organisms are slender branching Gram-positive bacilli embedded in the matrix of the granules. Similar structures can be formed by groups of other bacteria (Streptomyces, Staphylococcus and Streptococcus) or hyphae9). Aggregates of Nocardia occainsionally form granules similar to Actinomyces, though usually lacking the peripheral clubbing. Nocardia, however, differs from Actinomyces in that it is an aerobic and modified acid-fast positive organism.
Actinomycos is has been called ŌĆ£the most misdiagnosed disease ŌĆØ and there is ŌĆ£no disease which is so often missed by experienced clinicians ŌĆØ10). Actinomyces organisms are strictly anaerobic and will grow in culture only in anaerobic conditions. Also, the finding of Actinomyces by sputum cytology and/or culture, unless obtained directly from the bronchus, cannot be used as a definitive diagnosis due to its frequent presence as a commensal of the oral cavity.
The radiographic findings are dependent on the chronicity of the disease. In acute infections, the pattern has been reported as consisting of non-segmental air-space disease indistinguishable from other pneumonic process11). There is peripheral and lower lobe predominance, possibly reflecting the role of aspiration in the pathogenes is of this disease.
There are a few case reports of endobronchial actinomycosis. In two of the cases, endobronchial lesions resulted from extension of intra-pulmonary diseases12ŌĆō13). The other case, evaluating unresolved pneumonia, led to the diagnosis of endobronchial-lipoma with superimposed actinomycosis14). Common causes of endobronchial obstruction are bronchogenic carcinoma, bronchial benign tumors and endobronchial tuberculosis.
There are two case reports of endobronchial actinomycosis simulating endobronchial tuberculosis in Korea15,16). Ariel et al. reported five cases of endobronchial actinomycosis simulating bronchogenic carcinoma17).
The treatment of choice for infection with Actinomyces is penicillin. Since the microorganisms of the associated flora are not always susceptible to penicillin G, some investigators favor aminopenicillin +/ŌłÆ clavulanic acid1). Clindamycin, tetracyclines and erythromycin are alternative drugs in the case of penicillin allergy. In general, a treatment course of 3ŌĆō12 months is recommended for pulmonary actinomycosis, considering the difficult penetration of antibiotics in areas of dense fibrosis. High cure rates of actinomycosis on appropriate medical treatment have been reported in all recent series, with a mortality of only 1 out of 48 cases17).
In conclusion, endobronchial actinomycosis must be considered in the differential diagnosis of an endobronchial lesion, especially unresolving pneumonia. A presumptive clinical diagnosis should be attempted, since special requirements for successful culturing are needed. Fiberoptic bronchoscopy is a useful tool for diagnosis and may avoid surgical procedures. When sulfur granules are noted on routine histologic or cytologic examination of material obtained at bronchoscopy, additional stains will confirm the diagnosis of actinomycotic infection by revealing non-acid-fast filamentous micro-organisms stained positively with Gram stain.




 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





