INTRODUCTION
As a very common endocrine disease, thyroid nodules are occurred by 1ŌĆō7% according to the iodine uptake1,2) and their interpretation, whether malignant or benign, is a very important factor in treatment. The methods of differential diagnosis are clinical manifestation of thyroid nodules, ultrasonographic finding, isotope scanning finding and fine needle aspiration, of which the fine needle aspiration test is best for a cancer diagnosis with 85% of sensitivity and 90ŌĆō95% of specificity3). But, there are limitations on use because an inadequate or improper specimen often obstructs diagnosis and, moreover, it has a very poor sensitivity for a diagnosis of follicular thyroid cancer. As for the origin of thyroid cancer, various oncogenes and tumor suppressor genes are being studied, but it is still difficult to differentiate thyroid cancer from benign thyroid tumor by clinical application of them4ŌĆō6). Fra-1 is one of 4 Fos family (c-Fos, Fos B, Fra-1, Fra-2) forming AP-1 complex and it is known that its activity increases at a level of mRNA when transformed into a tumor cell, as well as in the test using thyroid cancer culture cell line7,8). According to the study using the pathologic thyroid tissue, expression of Fra-1 is absent in normal thyroid tissue, whereas it is present in thyroid cancer tissue, which suggested a possibility of use as a differential diagnosis of benign tumor and malignant tumor8). For a differential diagnosis of thyroid nodules, this study compared the expression of Fra-1 by the immunohistochemical (IHC) staining method in malignant thyroid tumor types, including papillary and follicular thyroid cancer, and benign thyroid tumor types, including adenomatous goiter and follicular adenoma.
MATERIALS AND METHODS
1. Subjects
4 types of thyroid tumor (18 cases of adenomatous goiter, 16 cases of follicular adenoma, 30 cases of papillary cancer and 10 cases of follicular cancer) confirmed by histologic diagnosis after operation were researched.
2. Methods
The expression of Fra-1 was measured by the immunohistochemical (IHC) staining method. First, the prepared paraffin tissue was put on a slide in a thickness of 4 ╬╝m and dried for over 2 hours at room temperature. It was washed in Xylene for 5 min. 3 times and then in alcohol of 100%, 95%, 80% for 2 min, in turn, to remove the paraffin. The slide was immersed in a 0.1 M Sodium citrate buffer and put in a microwave oven for 5 min. 2 times to expose the antigenecity, and then stabilized at room temperature. It was purified in a solution of pure methanol and 3% hydrogen peroxide mixed by 4:1 for 30 min to remove endogenous hydrogen peroxide. After the blocking antibody was treated for 30 min., it underwent the rabbit polyclonal Fra-1 (Santa Cruz, USA) as the primary antibody before incubation at 4┬░c for one night and then biotinylated goat anti-rabbit IgG (Vectastatin ABC kit, Vector, USA) as the secondary antibody for 30 min at room temperature. Thereafter it was reacted with ABC reagent for 30 min, stained with DAB (diaminobenzidin, DAKO, USA) for 5 min. and passed through hematoxylene control staining. After the dehydration course of alcohol and Xylene washing, it was made as a permanent slide with Permount solution. The expression of Fra-1 was evaluated by 4 scales from 0 to 3, which means no staining for 0 and the highest degree of staining for 3.
RESULTS
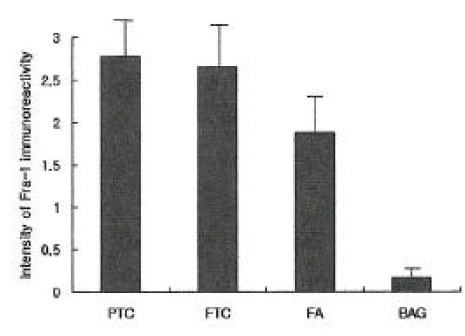
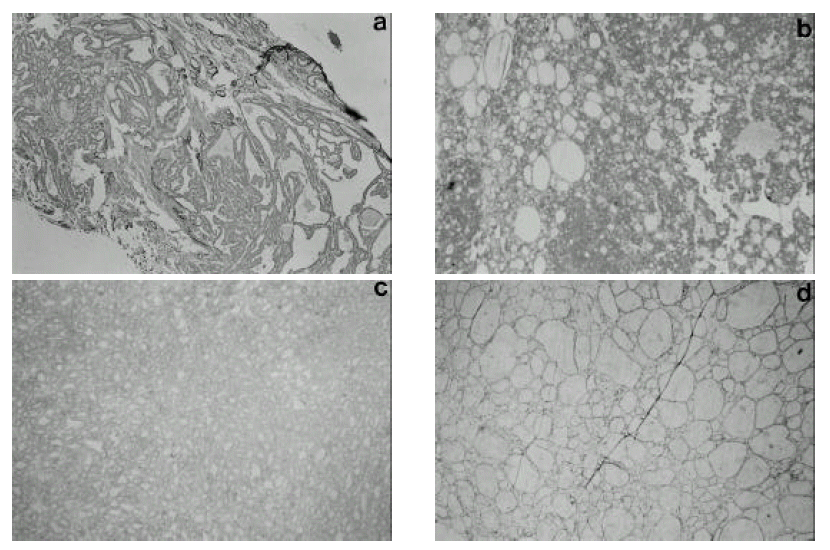
The expression of Fra-1 was stronger in papillary and follicular cancer than in benign thyroid tumor, but there was no difference in Fra-1 expression between the two types of carcinoma. Weak expression of Fra-1 was observed in all cases of follicular adenoma, and it was also weakly expressed in 6 out of 18 (33%) cases of adenomatous goiter (Figure 1).
DISCUSSION
AP-1 complex consists of 3 types of Jun family (c-Jun, Jun B, Jun D) and 4 types of Fos family (c-Fos, Fos B, Fra-1, Fra-2) and is related with activation of lots of genes, concerning regulation of cell-proliferation, and tumorigenesis, transformation by forming various kinds of homoor hetero-dimers9,10). As to tumorigenesis, the function of AP-1 complex became known for the first time when retro virus having V-jun and V-fos oncogenes was isolated and the function of oncogenes like c-Jun, Jun B, c-Fos, Fos B was clarified11ŌĆō14). On the other hand, it was reported that Jun-D negatively regulates fibroblast growth and partially antagonizes transformation by ras15). Some researches have been made about the function of AP-1 in transformation of mouse 3T3 and rat embryo fibroblast16ŌĆō18), and another research revealed that cultured cell from c-Jun-defective mouse resists against transformation by ras19). With regard to the role of AP-1 in the transformation of epithelial cell, the thyroid cell was used for the study7,8,25ŌĆō28) because increased AP-1 activity is essential for transformation of mouse epidermal cell20ŌĆō24), and thyroid cell transformation is a good model for neoplasm of epithelial cells. Among them, the test using rat thyroid culture cell line, including FRTL-5 and PC C1 3, reported that increment of AP-1 activity reflects the various changes of cell formation and Jun B and Fra-1 activity of AP-1 complex increases at a level of mRNA7). This increased Jun B and Fra-1 activity was known as being related to HMGI protein, which is known to present higher expression with malignant transformation of thyroid culture cells7,29). That is, among 3 HMGI proteins (HMGI, HMGY, HMGI-C), expression of HMGI-C protein is essential for Jun B and Fra-1 gene induction, which was suppressed in the cell line expressed anti-sense HMGI-C. When Fra-1 was suppressed by Fra-1 antisense RNA vector, malignant expression of transformed thyroid cells was reduced. So, Fra-1 gene was reported as important in cellular transformation pathways7). Seeing that regulation of Fra-1 transcription is highly dependant on AP-1 reactive inducer in the first intron of a gene and Fra-1 activity decreases in c-Fos or c-Jun deficient fibroblast, but increases by over-expression of another AP-1 family like FosB or c-Jun30), it can imply that increased Fra-1 expression involving oncogene is related to the activation of another AP-1 component made in the early stage of formation of transformation expression. Battista, et al.8) suggested that Fra-1 test using IHC staining method can be a help to differentiate thyroid cancer from benign thyroid tumor because Fra-1 stain was abundant in thyroid cancer but abscent in normal thyroid tissue from a result of study using IHC staining method to check the difference of Fra-1 expression, which was confirmed again by molecular biology technique and AP-1, especially Fra-1, c-Jun, Jun D, activity was also increased in cultured human thyroid cancer cells. This study was performed to evaluate if it is useful for a differential diagnosis of thyroid nodules by comparing the expression of Fra-1 between malignant thyroid tumor types, including papillary and follicular thyroid cancers, and benign thyroid tumor types, including adenomatous goiter and follicular adenoma. The expression of Fra-1 was much stronger in malignant thyroid tumor than in benign thyroid tumor, but there was no difference in Fra-1 expression between two types of carcinoma. In all the cases of follicular adenoma, the expression of Fra-1 was observed, though weak compared to thyroid cancers. In 6 cases out of 18 (33%) adenomatous goiter, Fra-1 was very weakly expressed. So the result revealed no specific staining reaction to the malignant thyroid tumor only, and it was not adequate to differentiate by the expression of Fra-1 whether malignant or benign the thyroid nodules are, as shown in the research of Battista, et al8). This is because the expression of Fra-1 can be essential for morphologic deformity, but not satisfactory for causing irreversible degeneration into a tumor, as mentioned in the experiment using rat fibroblast and thyroid culture cell by Bergers31) and Vallone, et al.32). In Fra-1 activity during the progression of neoplastic transformation, it is known that Fra-1 is a transcriptional goal of c-Fos and a temporary activity of c-Fos is essential for irreversible activity of Fra-17). The experimental model of skin tumor formation displayed the development of papilloma at c-Fos deficient mouse, but no progression of skin tumors33). Therefore, the possibility of a thyroid tumor induction at c-Fos deficient rat model and the research about the expression of Fra-1 at this time could be very helpful to clarify the role of Fra-1 in thyroid cancers. As a result, the expression of Fra-1 was stronger in thyroid cancer than in benign tumor, but IHC staining method appeared as irrelevant to differentiate malignant from benign tumors by the expression of Fra-1.



 PDF Links
PDF Links PubReader
PubReader ePub Link
ePub Link Full text via DOI
Full text via DOI Download Citation
Download Citation Print
Print





