A Case of Gastric Cancer Initially Presenting with Polydipsia
Article information
Abstract
Metastatic brain tumors from gastric cancer are extremely rare. A 61-year-old Korean woman, initially presenting with polydipsia and polyuria, was found to have metastatic lesions in the brain by MRI. We performed several diagnostic procedures to determine the origin of the brain metastases. She was revealed to have a soft tissue mass of the right adrenal gland and fungating ulcers in the stomach. Histologic studies of both the adrenal gland mass and gastric tissues revealed malignant tumors composed of anaplastic cells. Based on the electron microscopy study, the malignant tumor of the right adrenal gland was a metastatic lesion from the anaplastic carcinoma of stomach. Therefore, the malignant tumors of the brain were assumed to have originated from the gastric cancer.
This case report is presented to make clinicians aware of the possibility that diabetes insipidus (polydipsia) may present as an initial manifestation of brain metastases.
INTRODUCTION
Brain metastases are often multiple and simultaneous with metastases of other organs. The most frequent primary malignancy causing brain metastases is lung cancer, followed by breast cancer1,2).
Metastatic brain tumors from gastric cancer are extremely rare. Most gastric cancer patients with metastasis develop cachexia with peritoneal carcinomatosis and usually die of multiple organ failure. Single organ failure by metastasis is relatively uncommon as a cause of death, and brain metastasis itself rarely causes death.
Generally, the prognosis of patients with metastatic brain tumors is much poorer than that of patients with metastases in other organs, and the cases of metastases from gastric cancer are no exceptions3–5). Most patients who have metastatic brain lesions present with neurological symptoms during the course of their disease6).
We describe a 61-year-old Korean woman with brain metastases from gastric carcinoma, presenting with polydipsia and polyuria, which is very rare.
CASE REPORT
A 61-year-old woman was admitted because of weakness and polydipsia. Polydipsia suddenly began one month earlier, followed by progressive general weakness. She was initially evaluated at another hospital for the possibility of diabetes mellitus, but laboratory data were not diagnostic for the disease. She had no history of systemic disease or trauma. A physical examination showed her height to be 150 cm and body weight to be 50 kg. The chest and heart conditions were normal. A breast examination was also normal. The neurological examination on admission was normal. The laboratory tests on admission showed serum sodium at 152 mEq/L, serum potassium at 4.1 mEq/L, fasting blood glucose at 103 mg/dL, and hemoglobin A1c at 4.9%. The results of the liver and thyroid function tests, as well as the chest X-ray were all within normal limits. We performed the water deprivation test under the impression of diabetes insipidus. Urine osmolarity, after a pitressin injection, was markedly increased (>50%) and the results revealed complete pituitary diabetes insipidus (Table 1). A brain MRI was performed to demonstrate abnormal findings in the posterior pituitary gland, but it showed multiple enhanced nodules with surrounding edema in the entirety of the cerebral hemisphere, suggesting brain metastases (Figure 1). To determine the origin of the brain metastasis, we performed the following diagnostic procedures: mammography, abdominal CT scan, gastroscopy, and bone scintigraphy. The abdominal CT scan showed a 3.5-cm heterogenous mass of the right adrenal gland, which separated the kidney. The mass was suggested grossly as metastases and no other intra-abdominal mass was seen There was no abnormal finding detected by mammography. Two round-shaped, elevated lesions with central umblications were noticed on the antrum and low body of the stomach (Figure 2). We performed a percutaneous needle biopsy of the right adrenal mass. Histologic examinations of both the gastric tissue and right adrenal mass showed malignant tumors composed of anaplastic cells (Figure 3). Smears and a cell block from the right adrenal mass showed pleomorphic cells having bizarre nuclei and abundant eosinophilic cytoplasm, which were strongly positive for vimentin and weakly positive for cytokeratin immunostains, but were negative for synaptophysin. Thus, the right adrenal mass was suggested as a metastatic lesion from anaplastic carcinoma. The results of the immunohistochemical studies (cytokeratin, vimentin, desmin, smooth muscle actin, alpha-fetoprotein, and human chorionic gonadotropin) on the gastric tissues favored anaplastic carcinoma rather than sarcoma. The results of the c-kit from adrenal and gastric tissues were negative. The malignant cells of the adrenal mass were closely similar to the malignant cells from gastric tissues. Using an electron microscopy examination, the tumor cells of the gastric tissues were composed of a mixture of round oval cells and pleomorphic cells; and the tumor cells were occasionally joined by desmosome, which is suggested to be the origin of epithelial cells. However, there was no evidence of steroidogenic cells in the adrenal cortical carcinoma including in the well developed SER and mitochondria with tubular cisternae. The ultrastructural findings of the tumor cells were compatible with anaplastic carcinoma, including sarcomatoid carcinoma, rather than cortical carcinoma (Figure 4).Therefore, the metastatic lesions of the brain and right adrenal gland were assumed to have originated from gastric carcinoma. Further evaluation with bone scintigraphy showed multiple hot uptakes in the patients bones. She was managed with conservative treatment including desmopressin. After the desmopressin treatment, diabetes insipidus was greatly ameliorated. However, she died 3 months after the diagnosis.
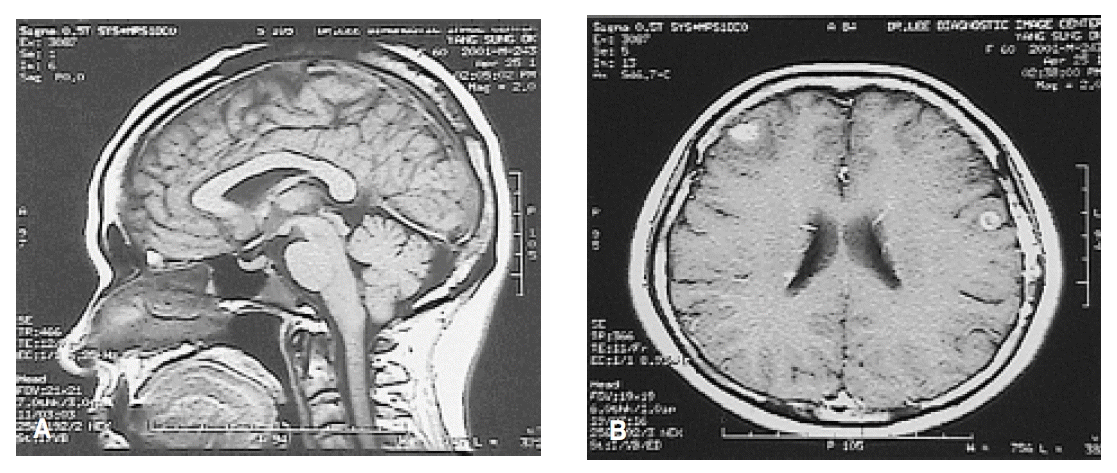
Brain MRI with Gadolinium enhancement. (1A) No visible bright signal intensity in the posterior pituitary gland. (1B) Multiple small round lesions with surrounding brain edema in the entire cerebral hemisphere.
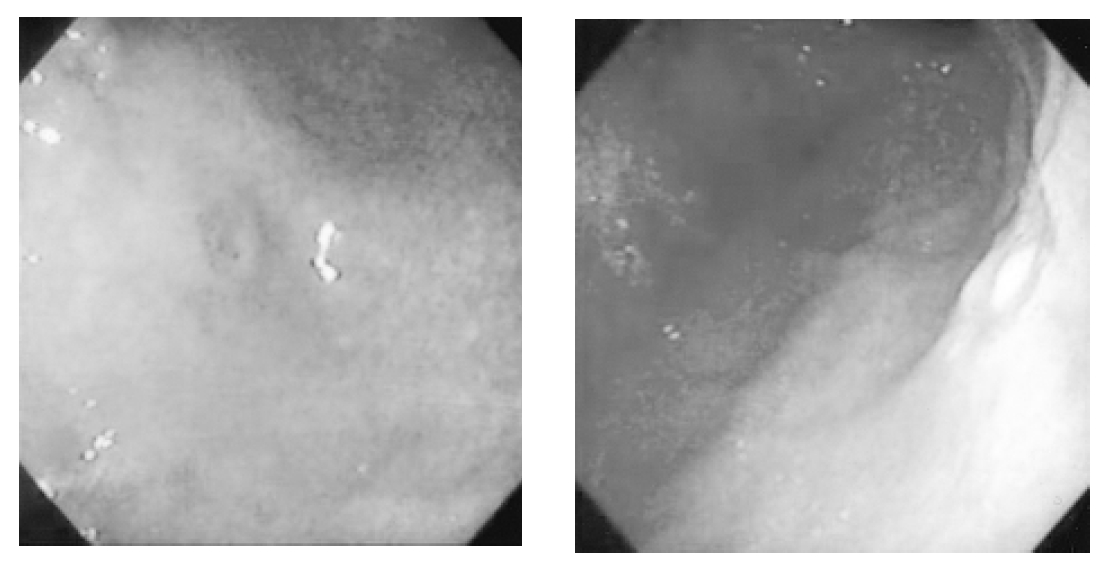
Two round-shaped elevated lesions with central umblications were noticed on the antrum (2A) and low body (2B) of the stomach by gastroscopy
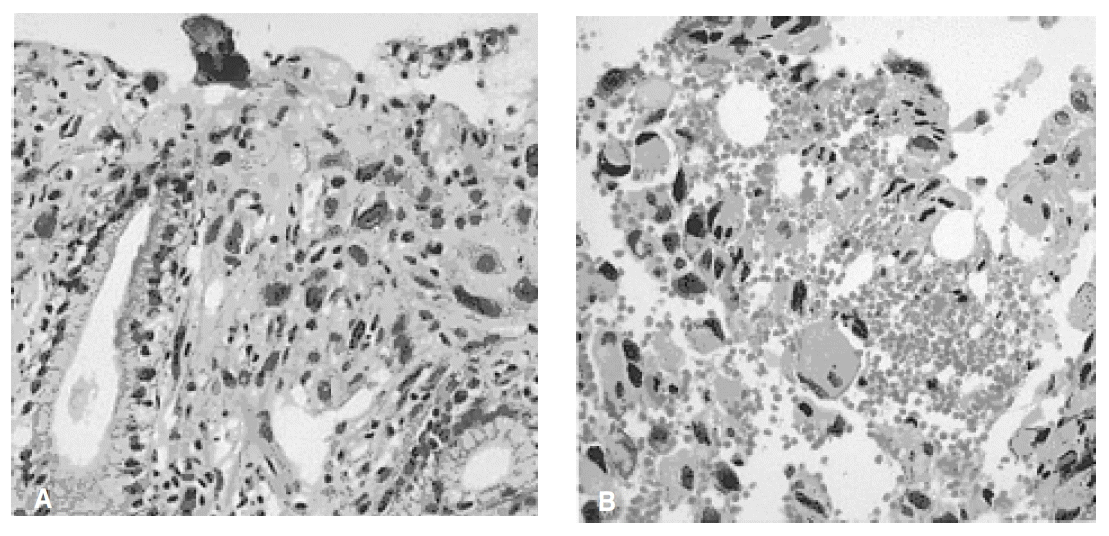
Photograph of the biopsied specimen of the gastric carcinoma. H&E (3A) Stomach : The tumor is mainly located in the submucosa and infiltrates the gastric mucosa. The tumor cells are large, polygonal in shape and show pleomorphic nuclei with frequent mitoses(H&E, ×100). (3B) Adrenal: The large and polygonal tumor cells have abundant eosinophillic cytoplasm and pleomorphic nuclei, which is similar to the gastric tumor (cell block, H&E, ×200).
DISCUSSION
Gastric carcinoma is one of the most common malignancies in Korea7), and most patients die from progressive cachexia6). Brain metastases from cancers of the gastrointestinal tract are uncommon, occurring in only 1.0–9.5% of all cases4). In a review of autopsies involving carcinomas, intracranial metastases were found in 8.3 of 1000 cases of gastric cancer 5). At M.D. Anderson, only 24 of 3320 patients with gastric cancer were found to have brain metastases during a 40-year period8). In Korea, Kim reported the rate of incidence of intracranial metastases from gastric carcinoma is 0.16%6). From a clinical perspective, brain metastases of gastric cancer have only been described in about 0.5–5% of cases9,10).
The most frequent primary malignancy causing brain metastases is lung cancer, followed by breast cancer1,2). Brain metastases from gastric cancer are unusual because the liver and lung act as barriers to the hematogenous spread of gastric cancer to the central nervous system. Accordingly, metastatic lesions located elsewhere have usually been found before the manifestation of the brain metastases11). The most common intracranial metastatic lesion of gastric cancer is meningeal carcinomatosis12,13), while isolated metastasis to the encephalic parenchyma is rare. Metastasis to the central nervous system from gastric cancer is mostly lymphogenous and occurs as meningioma, and metastasis to the cerebral parenchyma, which is generally thought to be via the arterial blood flow, is rare14).
According to a Japanese report, the most common presenting symptoms of brain metastases are weakness (67%), headache (42%), balance/gait abnormalities (42%), and nausea/emesis (38%)1). Less common findings are papilledema (13%) and seizures (8%). According to Kim’s report in Korea, patients with brain metastases present with headache, nausea, vomiting, cerebellar dysfunction, or other focal neurological deficits as the manifestations of parenchymal metastases6). But in the current case, the patient presented with polydipsia and polyuria without a headache and other neurological symptoms. We initially thought that she had central diabetes insipidus, and there was the possibility of breast cancer as the primary lesion. However, she was diagnosed with brain metastases by MRI.
The prognosis of metastatic brain tumors is generally poor15–18). In one study, median survival was 2.4 months (range: 5 days to 19.2 months)8). Many patients die soon after diagnosis, or they suddenly lose consciousness and are alternately found to have metastases by autopsy3–5). The patient of the current study died of complications related to tumor progression 3 months after the diagnosis of the disease.
In patients with brain metastases from gastric carcinoma, it appears that no currently available treatment regimen is likely to produce long-term survival or yield an improved neurological status19,20). In a review of studies about the efficacy of the treatment in metastatic gastric carcinoma, although a selection bias of surgical candidates obscured the true value of the surgical resection of a metastatic lesion, their recommendation is likely to be the best palliation when the condition of the patient is favorable to surgery8). In other Korean studies, neither chemotherapy nor radiation directed at the intracranial metastatic lesion altered the clinical course6). The patient of the current study did not receive a specific treatment for the brain lesions, except for a desmopressin treatment.
In this case, we used an EM examination to find the origin of the brain metastases, and found that the tumor cells of the gastric tissues were composed of a mixture of round oval cells and spindle cells. The tumor cells were occasionally joined by desmosome. However, there was no evidence of steroidogenic cells in the adrenal cortical carcinoma including in the well-developed SER and mitochondria with tubular cisternae. The ultrastructural findings of the tumor cells were compatible with anaplastic carcinoma, including sarcomatoid carcinoma, rather than cortical carcinoma. Finally, we conclude that the multiple metastatic brain lesion and right adrenal tumor were assumed to have originated from gastric carcinoma. This case report is presented to make clinicians aware of the possibility that diabetes insipidus (polydipsia and polyuria) may present as an initial manifestation of brain metastases of gastric carcinoma.

