The Clinical and Thyroid Function Studies of Lymphocytic Thyroiditis with Spontaneously Resolving Hyperthyroidism: Comparison to Subacute Thyroiditis
Article information
Abstract
Lymphocytic thyroiditis with spontaneously resolving hyperthyroidism (LT-SRH) has been reported in the past years, and is referred to as “silent thyroiditis.” It is characterized by a low or decreased radioactive iodine uptake (RAIU) of thyroid in a patient with hyperthyroidism in whom initial diagnosis is generally thought to be Graves’ disease.
Thirty-five patients who had hyperthyroidism or goiter with decreased RAIU have been assessed. Twenty-four (68.6%) of 35 patients had LT-SRH and the remaining patients, subacute thyroiditis (SAT).
The clinical characteristics of the patients with LT-SRH were a history of delivery, painless goiter, elevated T3 and T4 levels and positive anti-microsomal antibodies. Anti-microsomal antibodies were positive in 70.8% of the LT-SRH group, whereas 12.5% in the SAT group. Resolution of the hyperthyroidism took 8 to 12 months.
It is considered that LT-SRH is an autoimmune thyroiditis with spontaneously resolving hyperthyroidism and determination of the RAIU is very useful in differentiating from other forms of hyperthyroidism.
INTRODUCTION
Lymphocytic thyroiditis with spontaneously resolving hyperthyroidism (LT-SRH) is characterized by painless, nontender goiter, transient hyperthyroidism, decreased thyroid radioactive iodine uptake and focal or diffuse lymphocytic infiltration on biopsy specimen.
It had been classified LT-SRH as a variant of subacute thyroiditis (SAT), because the clinical course of each is so similar,1,2) However, Dorfman et al.3) and Nikolai et al.4) have reported LT-SRH was a similar form of disease as chronic lymphocytic thyroiditis (CLT) on the basis of the findings of positive thyroid auto-antibodies and lymphocytic infiltration on biopsy specimen. LT-SRH has been known so far as an autoimmune thyroiditis.
The importance in the differential diagnosis of hyperthyroidism with LT-SRH from other forms of thyrotoxicosis has been emphasized by other investigators, in order to avoid inadvertent treatment for this transient hyperthyroidism.5,6)
We present herein the clinical features, thyroid functions, thyroid RAIU and anti-thyroid antibodies as well as postpartum association in 24 patients with LT-SRH which was compared to SAT with or without hyperthyroidism. We emphasized that RAIU needed to recognize and differentiate from other forms of hyperthyroidism.
MATERIALS AND METHODS
Twenty-four patients with LT-SRH and 11 patients with SAT with or without hyperthyroidism, diagnosed inclusively between July, 1979 and June, 1983, have been investigated. The diagnosis of LT-SRH was made by the following criteria: (1) painless, nontender goiter, (2) elevated thyroxine (T4), triiodothyronine (T3) and free thyroxine (FT4) levels, and (3) depressed RAIU. The clinical diagnosis of SAT was made by the following criteria: (1) painful, tender thyroid gland, (2) fever, (3) elevation of the erythrocyte sedimentation rate (ESR), (4) normal or elevated serum T4, T3 and FT4 levels, and (5) decreased RAIU.
All 35 patients were asked about recent delivery, pregnancy and abortion history and iodine or thyroid hormone ingestion caused low RAIU were excluded.
The total white cell counts and ESR were done. Thyroid hormone concentrations were measured by competitive radioimmunoassay (RIA) with commercially available kits: T3, by Riabead diagnostic kit, T4 by Tetrabead-125 diagnostic kit and FT4 by Gammacoat kit.
The thyroid stimulating hormone (TSH) was measured by immunoradiometric assay with Htsh Riabead kit and anti-microsomal antibody and anti-thyroglobulin antibody by tanned erythrocyte hemagglutination technique with Fujirebio kit.
Thyroid scan and RAIU were performed in all patients at the time of the initial diagnosis.
RESULTS
Among 35 patients, twenty-four (68.6%) had LT-SRH and 11 patients (31.4%) SAT. All but one in the SAT group were female. The peak age incidence was 4th and 5th decades (73.0%) in SAT, and 3rd decade (62.5%) in LT-SRH group (Table 1).
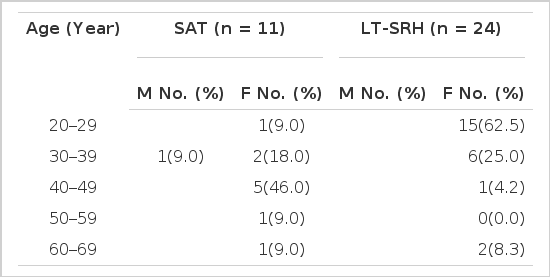
Age and sex distribution in patients with subacute thyroiditis and lymphocytic thyroiditis with spontaneously resolving hyperthyroidism
LT-SRH developed after delivery in 14 of 24 patients (58.3%) in LT-SRH group, but no patient with SAT had a recent history of delivery. Postpartum LT-SRH occurred within 4 months after delivery in 12 of 14 patients (85.6%) (Table 2).

The duration of first visit after delivery in 14 patients with lymphocytic thyroiditis with spontaneously resolving hyperthyroidism
The chief complaints on the first visit were fever (100%) and painful thyroid enlargement (63.6%) in SAT group and painless goiter (83.3%) and palpitation (20.8%) in LT-SRH group (Table 3).

The chief complaints in patients with subacute thyroiditis and lymphocytic thyroiditis with spontaneously resolving hyperthyroidism
Although the mean peripheral leukocytes counts were within the normal range and showed no significant difference between two diseases, the mean ESR was significantly high in the SAT group compared to the LT-SRH group, 39 ± 4 mm/h and 18 ± 3 mm/h, respectively (p<0.01). In all but one patient of the SAT group, ESR was more than 20 mm/h (Fig. 1).
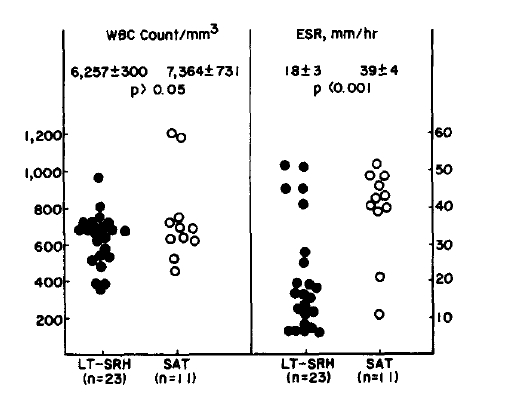
Peripheral blood leukocytes count and ESR values in patients with lymphocytic thyroiditis with spontaneously resolving hyperthyroidism and subacute thyroiditis with spontaneously resolving hyperthyroidism.
The values are expressed as mean ± SE.
Serum T3 and T4 values were increased in both diseases but serum T3 values in the LT-SRH group were significantly higher than subacute thyroiditis with spontaneously resolving hyperthyroidism (SAT-SRH) group (p<0.05). Mean T3/T4 (ng/ug) ratio of the patients with LT-SRH was less than 20 in all but 3 patients (Fig. 2).

T3, T4 and T3/T4 ratio in patients with lymphocytic thyroiditis with spontaneously resolving hyperthyroidism and subacute thyroiditis with spontaneously resolving hyperthyroidism.
The values are expressed as mean ± SE.
Normal values are within the dashed line.
Increased serum FT4 with low TSH values was observed in both diseases. Mean thyroid 24h-RAIU in LT-SRH and SAT group each was less than 2% and showed no significant difference between the two groups (Fig. 3).
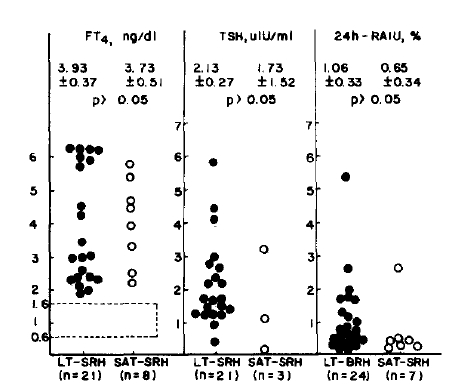
FT4, TSH and 24h-RAIU values in patients with lymopocytic thyroiditis with spontaneously resolving hyperthyroidism and subacute thyroiditis with spontaneously resolving hyperthryolism.
The values are expressed as mean ± SE.
Normal values are within the dashed line.
While only one patient (12.5%) in the SAT group had positive anti-microsomal antibodies in lower titer (1:102), 70.8% of LT-SRH group had positive anti-microsomal antibodies in relatively high titer. Nine patients (37.5%) of LT-SRH group showed positive anti-thyroglobulin antibodies, and they also had positive anti-microsomal antibodies. Although about 71% of patients with LT-SRH had positive anti-microsomal antibodies in a titer of more than 1:1602, 88.9% of the patients with positive anti-thyroglobulin antibodies showed positive reaction in a titer of less than 1:1602 (Table 4).

Antithyroid antibody titers in patients with subacute thyroiditis and lymphocytic thyroiditis with spontaneously resolving hyperthyroidism
The thyroid functions in the LT-SRH group returned to normal within 12 months after first diagnosis and 54.5% of patients were in hypothyroidism and 45.5% in euthyroidism on follow-up studies of 2 to 4 months.
The 24h-RAIU of thyroid 2 to 4 months later, showed increased uptake in 6 of 9 patients (66.7%) and of the remaining three patients, one was normal and two had decreased RAIU (Fig. 4 and 5).
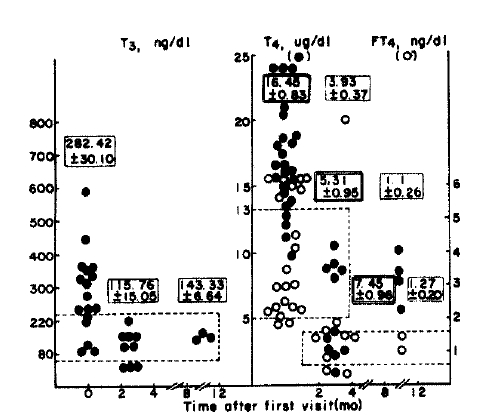
Results of serial T3, T4 and FT4 tests in patients with lymphocytic thyroiditis with spontaneously resolving hyperthyroidism.
The values are expressed as mean ± SE.
Normal values are within the dashed line.
DISCUSSION
LT-SRH is characterized by a spontaneously resolving hyperthyroidism, painless thyroid enlargment and decreased RAIU of thyroid, and it has been reported by several authors.7–13) LT-SRH has had numerous names, including hyperthyroiditis,7) thyrotoxicosis with painless thyroiditis,8) atypical subacute thyroiditis,9) lymphocytic thyroiditis with spontaneously resolving hyperthyroidism,10) hyperthyroidism with painless subacute thyroiditis,11) thyrotoxic lymphocytic thyroiditis12) and silent thyrotoxic thyroiditis.13)
The thyrotoxicosis which can raise from Graves’ disease, toxic multinodular goiter and toxic adenoma, is due to increased synthesis and release of thyroid hormones from the thyroid gland. However, LT-SRH and SAT-SRH are characterized by inflammation and alteration of the normal thyroid architecture, that produce hyperthyroidism because of the release of preformed stores of thyroid hormone and they have a similar clinical course to each other.13)
Volpé et al.14) have described four stages of thyroid gland function of SAT. In the first stage, acute inflammation causes preformed stores of thyroid hormones to be released from the inflammed thyroid gland. Patients show signs of clinical hyperthyroidism and thyroid RAIU is absent or quite low. In the second stage, over a period of a few weeks, serum levels of thyroid hormones decline to normal and clinical evidence of hyperthyroidism ceased. In the third stage, serum thyroid hormone values decline to hypothyroid level, serum TSH rises to above normal levels, RAIU rises to normal or increased levels and the patients may be clinically hypothyroid. The fourth stage is characterized by a return of all thyroid functions to normal.
The T3/T4 (ng/ug) ratio by Amino et al.15) separated destructive-induced hyperthyroidism (ratio<20) from stimulation-induced hyperthyroidism (ratio>20) and this ratio was shown as a useful tool in differentiating these forms from Graves’ disease. Mean T3/T4 ratio in our study of SAT-SRH and LT-SRH groups, were 13.68 ± 0.73 and 15.37 ± 1.12, respectively, which supported a destructive-induced hyperthyoidism.
In other literature, they have reported a peak age incidence from the 3rd to 5th decades and the sex ratio (male to female) was 1:1 to 1:4 in the patients with LT-SRH except 8 female patients who were described by Dorfman et al.3)
It has been reported that the prevalence of LT-SRH related to delivery was low. Nikolai et al.10) reported that 5 of 56 patients (8.9%) of LT-SRH group had the relation to delivery. Also only 5 patients by Ginsberg and Walfish16) and only 1 patient by Daily17) were reported. On the contrary, LT-SRH in more than half of our patients had occurred after delivery.
Amino et al.18,19) in Japan, Huh et al.20) in Korea, and Fein et al.21) in U.S.A. reported autoimmune thyroiditis which showed postpartum transient hypothyroidism with increased thyroid RAIU, and they suggested the possibilities that it is similar or the same as postpartum LT-SRH,19,21,22)
It is well known that autoimmune thyroiditis shows improvement during pregnancy which brings about the modification of cellular immunity and that would be aggravated after delivery.
It seems that the prevalence of the autoimmune thyroiditis related to delivery is higher in oriental countries than western countries.
Although the results of thyroid hormone tests, TSH and thyroid RAIU demonstrated similar distribution in both diseases, serum T3 was significantly higher in the patients with LT-SRH group than those with SAT in our study. Nikolai et al.4) were not able to find any difference in serum thyroid hormone values between two diseases, and it was thought to be difficult to differentiate LT-SRH from SAT only by the thyroid hormone values.
The coexistence of hyperthyroidism with suppressed RAIU could be seen in the patients with excessive iodine intake, surreptitious thyroxine ingestion, or the rare case of ectopic thyroid hormone production from struma ovarii and other tumors as well as LT-SRH and SAT-SRH.
The patients in hyperthyroid stage of LT-SRH excreted the increased urinary iodine as seen in iodine-induced thyrotoxicosis9,10,23) and TSH-stimulatioin test could be helpful in differentiating two forms of hyperthyroidism.24) However, we supposed that Iodine-induced thyrotoxicosis could be excluded only by careful history taking.
All patients with postitive anti-thyroglobulin antibodies in the LT-SRH group, also had anti-microsomal antibodies. In our study, 37.5% of patients had anti-thyroglobulin antibodies, and 70.8% of the patients including above 37.5%, had anti-microsomal antibodies in the LT-SRH group, that was similar in result as postpartum transient hypothyroidism.19) These supported that the anti-microsomal antibody test is more accurate than anti-thyroglobulin antibody test in the diagnosis of LT-SRH.
The focal and diffuse lymphocytic infiltration in biopsy specimen from LT-SRH that is similar to CLT was reported by many authors.8,12,23,25)
Gorman et al.23) pointed out lack of oxyphilic change, suggested histologic difference from CLT, but Nikolai et al.10) found focal oxyphilic changes in the biopsy specimens of LT-SRH too. It is difficult to confirm the diagnosis of LT-SRH only by fine needle aspiration cytology of the thyroid gland,26,27) but we could find numerous lymphocytes in 4 patients of the LT-SRH group.
The occurrence of SAT is frequently preceded by a viral respiratory infection and anti-thyroid antibodies could be present in only a minority of patients, which is due to secondary release of antigenic materials from thyroid associated with viral infection.28,29) However, anti-thyroid antibodies are found in a high percentage of patients with LT-SRH. Dorfman et al.3) had reported that LT-SRH was the same disease as CLT, on the basis of the observation of positive anti-thyroglobulin antibodies by RIA in all patients with LT-SRH.
Although it is unable to distinguish LT-SRH from CLT by anti-thyroid antibody test and biopsy, we can diagnose CLT with hyperthyroidism without difficulty because the thyroid RAIU shows normal or elevated values in contrast to LT-SRH.30)
The clinical course of LT-SRH is not certain. Nikolai et al.4) observed 54 patients with a history of LT-SRH in 1 to 15 years follow-up, 23 patients (42.6%) were found to have a goiter after returning to normal thyroid hormone levels and persistent lymphocytic infiltration were noted on biopsy specimen. Also 5.6% of patients showed permanent hypothyroidism and 32% of them had persistent antithyroid antibodies and 11% of patients experienced recurrence. These findings suggested that LT-SRH is a persistent and progressive disease as CLT with recurrent episodes, so the development of goiter and thyroid failure is a natural fate.
We could observe the serum thyroid hormone levels in 5 patients with LT-SRH which returned to normal range within 12 months, but we should follow them up at a regular interval.
The possibility that LT-SRH is thought to be one form of autoimmune thyroiditis is cited primarily because LT-SRH is more frequent in women and especially during the postpartum period. The relatively high percentage of the patients who have antithyroid antibodies and diffuse lymphocytes infiltration are found in biopsy specimen.
The clinical features such as a recent history of delivery, thyroid pain, fever, and elevation of ESR, positivity of anti-thyroid antibodies and the findings of the thyroid biopsy could be used to differentiate LT-SRH from SAT.
Thyroid RAIU determination is very important in differential diagnosis from other forms of hyperthyroidism.
It is thought that more studies such as iodide metabolism and immunologic studies will be needed to find out the precise pathogenesis of LT-SRH.
