Neutrophil Chemotactic Activities In Bronchoalveolar Lavage Fluid From Patients with Bronchial Asthma
Article information
Abstract
Objectives
To elucidate the presence of neutrophil chemotactic factor (NCF) and characterize them in the bronchial trees of symptomatic patients with bronchial asthma.
Methods
Bronchoalveolar lavage (BAL) fluids were concentrated by ultrafiltration. Differential counts of BAL cells was performed upto 500 cells on the cytocentrifuge-prepared slides. NCF activities in concentrated BAL fluids were measured by using microchemotactic chamber.
These NCF activities were characterized by heat-stability, sensitivity to try sin and solubility into organic solvent.
Results
NCF activities were significantly higher in low molecular weight (LMW)-BAL fluid fraction below 5000 dalton than those in high molecular weight (HMW)-BAL fluid fraction. The NCF activities were significantly higher in the patients with bronchial asthma when compared to those of normal subiects. The LMW-NCF and HMW-NCF activities were correlated with the percentages of neutrophils in BAL fluid in the patients with bronchial asthma. The LMW-NCF activities were extractable into ether, stable to heat and resistant to trypsin.
Conclusions
Main NCF activities in BAL fluid are suggested to be lipid substances with low molecular weight less than 5000 dalton and these substances may recruit neutrophils into the bronchial trees of patients with bronchial asthma.
INTRODUCTION
Bronchial asthma has been characterized as an inflammatory disease in the airway walls1,2). Several kinds of inflammatory cells have been reported to play an important role in initiation and amplificatiuon of inflammation leading to the increase of bronchial hyper-reactivity in patients with bronchial asthma3,4) and in animal models5–7).
Although eosinophils are the major effector cells leading to bronchospasm and inflammation, neutrophils have also been thought to play an important role in the pathogenesis of bronchial asthma. Direct examinations of bronchoalveolar lavage (BAL) fluids, after antigen challenge5,6,8,9) and after exposure to environmental chemicals such as ozone7) and toluene diisocyanate (TDI)10), showed marked increase in the number of neutrophils. The change in the number of neutrophils was correlated with the magnitude of bronchial hyper-reactivity and the late allergic reaction in several species5,6)
For the emigration of neutrophils into the bronchial trees, NCF should be produced and present in the bronchial trees. Heat stable NCF of high molecular weight has been characterized in the circulation of patients with bronchial asthma when they were in status asthmaticus11) or when provoked by related antigens12), exercise13) or TDI14). This NCF is suggested to be derived from mast cells. However, other inflammatory cells produce different types of NCF from those of mast cells, and mast cells also produce other types of NCF than the high molecular weight-NCF15). Thus, the direct measurement and characterization of NCF in the bronchial trees of patients with bronchial asthma, is important to reveal out the nature of NCF which might initiate and sustain the bronchial inflammation.
We tried to reveal the presence of NCF in BAL fluid of the patients with bronchial asthma, andpartially characterized these activities and examined the correlation with the number of neutrophils in BAL fluid and parameters of airway obstrcution.
MATERIALS AND METHODS
1. Study Population
This study was approved by the Soonchunhyang University Human Subject Committee. All subjects gave informed consent before participation. Nineteen patients were included in bronchial asthma and nine healthy persons were included in normal control group, they were non-smokers. Their clinical charcateristics are described in Table 1 and here. Allergic rhinitis was associated in 5 cases among nineteen patients with bronchial asthma. The onset of the bronchial asthma varied from one to twenty-three years ago. The presence of atopy was determined by the presence of immediate wheal and flare skin reactions to more than one of common allergen extracts among 50 allergens (Bencard, England) in Korea. The reaction was regarded as positive when wheal size by allergen extract was greater than that by 0.1% histamine. Specific IgE, measured by radioallergosorbent test(RAST) to Dermatophagoides farinae and pteronyssinus, was positive in 4 patients with atopic asthma.
The pattern of asthmatic attack was acute intermittent in all patients, except two cases of non-atopic asthma and one case of atopic asthma who had a chronic persistent pattern of bronchospastic attack. Acute intermittent pattern was defined to have episodes of shortness of breath and wheezing without persistent symptoms between episodes, and chronic persistent to have persistent symptoms or derangement of lung function test even between episodes of acute asthmatic attack. No patients had evidence of respiratory bacterial infection within 4 weeks of the study.
2. Measurement of Pulmonary Functional Parameters and PC20
FVC, FEV1 and PEFR were measured with Fukuda-300(Japan) without previous medication, including aminophylline, for the twelve hours before lung function measurement. PC20 of histamine was measured by Cockroft’s method16) from the concentration of 0.025mg/ml to 25mg/ml of histamine. These parameters were measured two days before the BAL procedure.
FEV1 and PEFR were significantly lower in the patients with non-atopic bronchial asthma than those of normal subjects. PC20 was significantly lower in patients with non-atopic and atopic bronchial asthma than that of normal subjects. Sex was matched and age was not significantly different between the three groups. Physiologic parameters and duration of disease were not different between atopic and non-atopic bronchial asthma.
3. Bronchoalveolar Lavage
In 7 cases of the patients, BAL was carried out without medication, except for two puffs of bronchodilator before the procedure. In 12 cases of the apparently bronchospastic patients, BAL was carried out, when the patients became tolerable to bronchoscopy after treatment of the last acute bronchospastic attack, for two weeks with aminophylline and beta-stimulants. Beta-stimulants were stopped at least one week before bronchoscopy. Aminophylline was also stopped at least 24 hours before the BAL procedure. None of the patients had ever used disodium chromoglycate and inhalant steroids before the procedure. All study subjects had 2 puffs of bronchodilator inhaled, and atropin and diazepam administered as premedication before bronchoscopy. The BAL procedure was performed by one person with instillation of 4 times aliquots of 50ml normal saline into right middle lobe under fiberoptic bronchoscopy as previously described by NHLBI workshop summaries17).
4. Isolation of Neutrophils and Chemotaxis Assay
Neutrophils were obtained from heparinized whole blood of one AB type normal volunteer by sedimentation on 6% dextran-dextrose solution, centrifugation on Ficoll-Hypaque solution (specific gravity of 1.077) and hypotonic lysis18). Cells containing neutrophils more than 95% were suspended in Hank’s balanced salt solution (HBSS) with 0.4% of bovine serum albumin (BSA) at the concentration of 1 × 106 cells/ml.
The chemotactic assay was developed with a 48 well microchemotactic chamber (Neuro probe, Md., USA) in which upper and lower chamber were separated by a polycarbonate filter with 5 μm sized pores (Nucleopore Co., Cal., USA). All the assays were performed in a blind fashion by one person. The chambers were filled with 25μ 1 of solution to be tested and covered with filter. 45μ 1 of the cell suspension was deposited on top of the filter. The chamber was incubated for 90 minutes at 37°C in a humidified incubator containing 5% CO2. Thereafter, the filter was removed, fixed in 100% methanol and subsequently stained with Diff Quick stain solution. The number of neutrophils which migrated trough the filter was determined microscopically by 40 × objective. Ten fields were counted per well and all experiments were conducted in quadruple. The results were expressed as percentage of neutrophils migrated, which was calculated as the mean number of neutrophils per field in experimental wells divided by that in negative control wells times 10019). 10−5mole on N-formyl-methyl-leucine-phenylalanine (f-MLP) was used as positive control and HBSS with BSA as negative control. The intra-assay coefficient of variation and inter-assay coefficient of variation were less than 20%.
5. Partial Characterization of the Chemotactic Factor
BAL fluid was fractionated and concentrated 10 times as the low molecular weight (LMW) and high molecular weight (HMW) by using a stirred cell equipped with YM-5 filter membrane (Amicon Co., MA., USA) with a cutoff point of 5000 dalton. The intially filtered one tenth volume was defined as LMW. The heat stability of the NCF in LMW-BAL fluid was evaluated by heating for 30 minutes at 56°C and 100°C in 5 cases of bronchial asthma.
The sensitivity of NCF to trypsin was evaluated by incubating the LMW-BAL fluid with 10μg/ml of trypsin for 30 minutes at 37°C. After incubation, the enzyme was inactivated by heating for 15 minutes at 100°C.
The solubility of LMW-NCF in organic solvent was evaluated by incubating 5ml of LMW-BAL fluid with an equal volume of ethylacetate for 1 hour and extracting. After an additional extraction into ethylacetate, the organic solvent was dried with a stream of nitrogen and the extracted material was resuspended in 5 ml of HBSS and adjusted to pH 7.4.
The differences between groups were compared using the non-parametric Kruskal-Wallis H test for continuous data and, if found significant, the Mann-Whitney test with Bonferroni’s correction was applied to compare any two groups. Wilcoxon signed-rank test was used for paired samples. The relationships between NCF activities, physiologic parameters and neutrophil percentages in BAL fluids were studied using Spearman’s rank correlations. The difference was considered significant when p value was less than 0.05.
The results are expressed as mean ± standard error unless otherwise stated.
RESULTS
1. NCF Activity in BAL Fluid according to Molecular Weight
The results shown in Table 2 reflect the magnitude of chemotactic activity produced by the serial dilutions of concentrates of BAL fluid from 6 patients with bronchial asthma. When 10 times concentration of LMW-BAL fluid was used, 1:5 dilutions (20%) in the lower chamber exhibited minimal NCF activity, statistically insignificant compared with negative control. Whne 1:2.5 dilutions (40%) and 1:1.25 dilutions (80%) were used in the lower chamber, NCF activities were significantly greater than that of negative control comprising HBSS and BSA (p<0.05). On the other hand, concentrates of LMW-BAL fluid in the upper chamber tended to show negative NCF activities. 80% concentrates of HMW-BAL fluid showed the enhanced NCF activity, but this change was statistically insignificant when compared with that of negative control.
2. Comparision of NCF Activity in BAL Fluid between Asthma and Control
In LMW-BAL fluid of the normal subjects, only 2 among 9 cases exhibited greater NCF activities than negative control with the value of 110% and 154%. On the other hand, 17 among 19 patients with bronchial asthma exhibited greater NCF activities in LMW-BAL fluid than negative control (Fig. 1). In HMW-BAL fluid, 10 among 19 patients with bronchial asthma showed greater NCF activities than that of negative control containing HBSS-BSA (Fig. 1).

NCF activity in low (LMW) and high molecular weight (HMW)-BAL fluid fractions from the patients with atopic bronchial asthma (triangle), non-atopic bronchial asthma(closed circle) and normal subjects (open circel).
* p<0.05 vs normal control
** p<0.05 vs HMW of bronchial asthma
The mean value of NCF activities was significantly greater in LMW-BAL fluid from the patients with bronchial asthma than that of normal subjects (170.9 ± 19.0% vs 100 ± 7.25%, p<0. 005). However, the mean value of NCF activities was not higher in HMW-BAL fluid concentrates than from those with bronchial asthma when compared with that of normal subjects (111.3 ± 11.5% vs 88.6 ± 3.9%, p = 0.063). Atopic bronchial asthma had the greater magnitude of NCF activitie than those of non-atopic bronchial asthma in LMW (208.3 ± 41.1% vs 143.8 ± 11.4%, p = 0.27) and HMW (123.9 ± 24.7% vs 103.7 ±10.5%, p = 0.41) of BAL fluid. However these differences were not statistically different. The non-medicatied patients with bronchial asthma had the same NCF activities as the medicated patients with bronchial asthma in LMW-BAL fluid (160.1±18.8% vs 177.3 ± 27.6%, p>0.05) and HMW-BAL fluid (107.0 ± 12.6% vs 113.8 ± 16.5 %, p>0.05). The NCF activities in LMW-BAL fluid were significantly higher than those in HMW-BAL fluid in the patients with bronchial asthma (Fig. 1).
The NCF activities in LMW-BAL fluid correlated with those in HMW-BAL fluid when observed in the entire study populations (r = 0.79. p<0.001, n = 28) as well as the asthmatic group (r = 0.79, p<0.001, n= 19. Fig. 2)
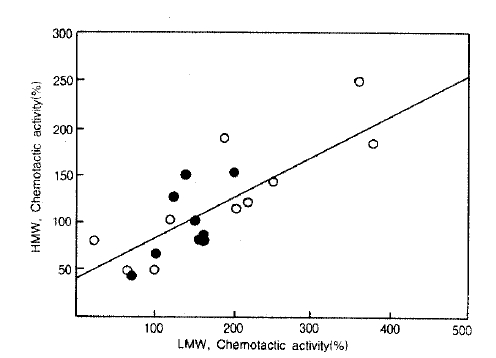
Relationship of chemotactic activity between HMW and LMW-BAL fluid. Linear regression analysis revealed significant correlation between chemotactic activities of HMW and LMW-BAL fluid when observed in the patients with atopic (triangle)and non-atopic (closed circle) bronchial asthma(r=0.79, p = 0. 001, n=19).
3. Correlation of NCF activity with physiologic Changes
NCF activities in LMW-BAL fluid(107.0 ±12.6 % vs 113.8 ± 16.5%) The NCF asdivities in LMW-BAL fluid were inversely correlated with PEFR (r = −0.370, p = 0.026, n = 28) and PC20 (r = −0.40, p = 0.03, n = 28) when observed in whole study populations, including normal subjects and the patients with bronchial asthma. However, the correlations between these parameters became statistically insignificant when observed only in the patients with bronchial asthma.
4. Correlation of NCF Activity with Percentage of Neutrophils in BAL Fluid
To assess whether the NCF activities in BAL fluid had an effect on emigration of neutrophils into the bronchial tree, we compared the NCF activities in LMW and HMW-BAL fluids with the percentage of neutrophils recovered in BAL fluid.
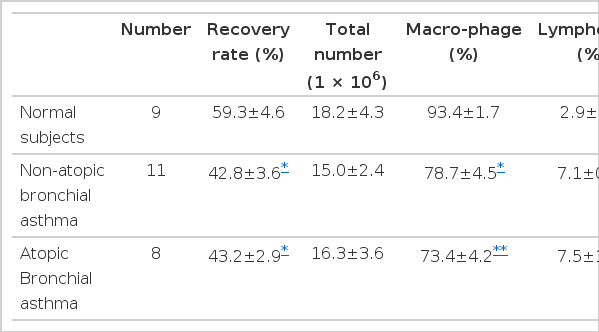
The recovery rate was lower in non-atopic, as well as atopic patients with bronchial asthma, when compared with that of normal subjects. Total number of cells recovered in BAL fluid was not different between each group of bronchial asthma and normal subjects. In cellular composition, the atopic and non-atopic patients with bronchial asthma had significantly greater percentages of lymphocytes, neutrophils and eosinophils than those of normal control subjects. Otherwise, the percentage of macrophages was significantly lower in BAL fluid from the patients with bronchial asthma when compared with that of normal control subjects. Cellular composition was not different between the patients with non-atopic and atopic bronchial asthma (Table 3).
NCF activities in LMW were significantly correlated with the percentage of neutrophils in BAL fluid, when all study subjects were included (r= 0.63, p = 0.001, n = 28; Fig. 3), as well as when ovserved in only the bronchial asthma group (r= 0.59, p = 0.004, n=19; Fig. 3). HMW-NCF activities in BAL fluid also showed significant correlation with the percentage of neutrophils when observed in the asthma group (r = 0.49, p = 0.017, n=19) as well as in the entire study subjects (r = 0.51. p = 0.003, n = 28).
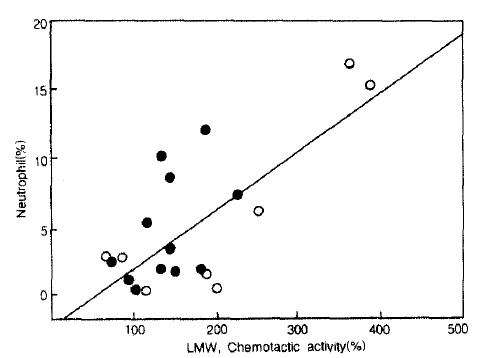
Relationship between NCF activities in LMW-BAL fluid with the percentage of neutrophils in BAL fluid. Linear regression analysis revealed significant correlation when observed in the patients with atopic (triangle) and non-atopic (aclosed circle) bronchial asthma (r=0. 59, p = 0.004, n= 19).
The percentage of neutrophils was also significantly correlated with PEFR (r = −0.381, p = 0.023, n = 28) and showed the tendency to correlate with PC20 (r = −0.287, p = 0.087, n = 24) when observed in entire study subjects. However, these correlations became insignificant when observed only in the patients with bronchial asthma.
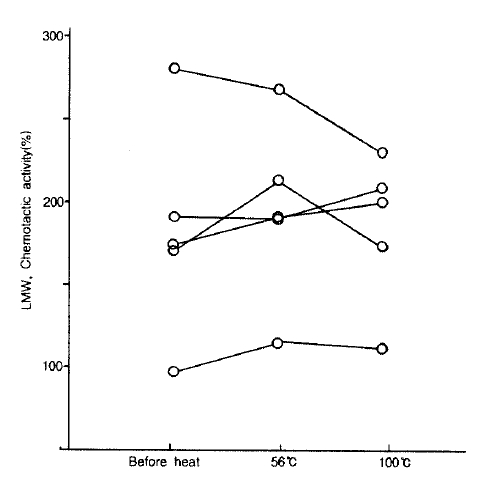
5. Effect of Heat on NCF Activity in LMW-BAL Fluid
We evaluated the effect of heating on the NCF activities in 5 cases of the bronchial asthma group. In all cases, the change in NCF activities by the heat treatment was negligible. The mean value after 56°C for 30 minutes was the same as that before heat inactivation (195.4 ± 22.0% vs 182.9 ± 26.1%, p>0.05). The heat inactivation with 100°C for 30 minutes also had no effect on NCF activities, when compared with those before heat treatment (184.8 ± 18.3% vs 182.9 ± 26.1%, p>0.05. Fig. 4).
6. Effect of Trypsin and ether Acetate on NCF Activities in LMW-BAL Fluid
The effect of trypsin on NCF activities in LMW-BAL fluid was evaluated in 5 cases of patients with bronchial asthma. The mean value of NCF activities was not changed by treatment with trypsin (175.2 ± 17.2% vs 186.6 ± 16.1%, p<0.05. Fig. 5).
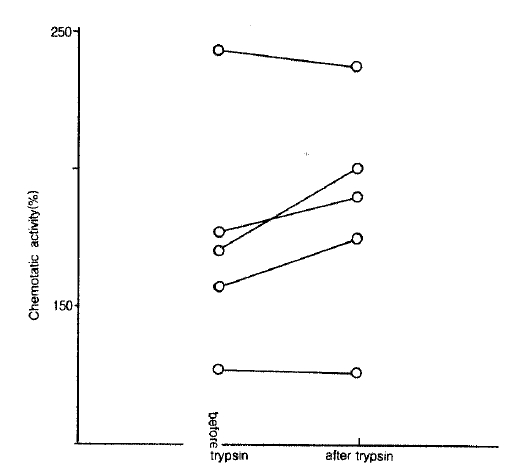
The effect of trypsin on NCF activities in LMW-BAL fluid. The NCF activities did not change after trypsin treatment for 30 minutes at 37°C.
Ether extraction was done using LMW-BAL fluid from 5 patients with bronchial asthma. Extracts contained greater NCF activities when compared with those in LMW before extraction (249.0 ± 26.5% vs 185.3 ± 4.3%, p<0.05) and extract remnants contained lesser NCF activities compared to those in ethyl extracts (14 4 ±11.5 % vs 249.0 ± 26.5%, p<0.01) and samples before extraction (Fig. 6).
DISCUSSION
In this investigation, we demonstrated NCF activities in the bronchial fluid of patients with bronchial asthma. Its potency was the same between the patients with atopic and non-atopic bronchial asthma. In addition, NCF activities were closely correlated with the number of neutrophils recovered in the bronc-hoalveolar lavage. The fact that NCF activities were absent in BAL fluid of normal control subjects is consistent with the observation that neutrophils are rarely found in the bronchial trees of normal individuals. These findings suggest that these NCF activities play a role in modulation the migration of neutrophils into the bronchial trees of patients with bronchial asthma.
There are some evidences of neutrophils activation in bronchial asthma. Expression of complement receptor on neutrophils was elevated during early and late reactions after antigen inhalation20) and in the patients with chronic bronchial asthma21). Animal studies also support the role of granulocytes in airway inflammation and bronchial hyperreactivity10,22).
A number of neutrophils, specific chemoattractants relevant to allergic diseases, have been identified15). They include C5a, C5a des arg derived from complement, LTB4, PAF, IL-123) and IL-824), heat stable-chemotaxin with molecular weight of 10kd secreted by macrophages25). However, the precise factors that direct neutrophils accumulation into the airway of bronchial asthma has been obscure until now.
High molecular weight-NCF has been identified in sera from the patients with bronchial asthma in status, asthmaticus11) and induced by antigen exposure12), exercise13) or TDI14). In all of these studies, the principal activity was associated with proteins having a molecular size of approximately 600 kd and an isoelectrical point of approximately 6.5. However, this high molecular weight-NCF has not been reported to be present in the bronchial tree of patients with bronchial asthma.
Because any NCF activity could not be checked when BAL fluid was used in unconcentrated form (data not shown), we concentrated BAL fluid by ultrafiltration membrane with the cutting off point of 5000 dalton. NCF activities were found in both LMW and HMW-BAL fluid in some patients with bronchial asthma. Seven of nineteen patients with bronchial asthma had a greater NCF activity in HMW-BAL fluid than 110% of negative control. We did not check whether this NCF activity is the same substance as mast cell derived-high molecular weight-NCF or not. However, NCF activities were found more frequently in BAL fluid fraction of molecular range below 5000 dalton and the potency was also greater in the low molecular range when compared to that above 5000 dalton in our study. These findings mean that NCF activity is mainly low molecular weight substances in the bronchial tree of patients with bronchial asthma.
The drugs used in our study before bronchoscopy were aminophylline and beta-stimulants, which are potent inhibitors for mast cells to release the products. However, the patients with bronchial asthma, who received the medication, had the same NCF activity as the non-medicated patients. These drugs were found not to inhibit neutrophil locomotion11).
The NCF activities in the bronchial tree can be derived from several kinds of inflammatory cells such as macrophages26), lymphocytes and mast cells or from exudated serum into the bronchial tree. The main NCF activities in LMW-BAL fluid does not seem to be related to any serum-derived chemotactic factors. First, it was not inactivated by heating for 30 minutes, 56°C and 100°C. Second, NCF activity was present predominantly in a molecular weight range below 5000 daltons. These findings can distinguish NCF in LMW-BAL fluid from the serum-derived chemotactic factors that are generated as a result of activation of the complement system, hageman factor or the kinin generating system. IL-1 and IL-8 could be excluded by the nature of heat stability and resistance to trypsin of NCF in LMW-BAL fluid. However, it is unknown that NCF activity in HMW range of BAL fluid is IL-1 or IL-8.
The main NCF activity in LMW-BAL fluid was hydrophobic, as indicated by its preferential solubility in lipid solvent and resistance to treatment with trypsin. This result indicates that at least a major portion of NCF activities in LMW fraction appeared to be lipid substance. Among the lipid substances, LTB4 and PAF have NCF activity with the molecular weight less than 500 dalton. LTB4 is a potent pro-inflammatory mediator27) with a wide variety of biologic activities, including smooth muscle contraction, mucus hypersecretion and leukocyte activation, which may be of particular relevance to the pathology of bronchial asthma. The concentration of LTB4 was reported to be significantly elevated in BAL fluid of the symptomatic patients with bronchial asthma28). Alveolar macrophage also produced LTB4 through IgE-dependent process in the patients with bronchial asthma29).
PAF is another lipid substance recognized as one of the most potent mediators in bronchial asthma. PAF is able to elicit bronchospasm, mucosal edema and inflammatory cell recruitment, inducing the increase in bronchial reactivity, which may last up to 14 days30). The presence of PAF was also confirmed in BAL fluid of the patients with bronchial asthma31). As an alternative method to characterize the nature of NCF, deactivation study with known substances, such as LTB4 and PAF, has been used. However, in our study, this method was not tried.
In conclusion, our study suggests that airway inflammation with neutrophils may be induced by the presence of NCF activities with low molecular weight-lipid substances in the bronchial tree of patients with bronchial asthma.
Acknowledgments
The study was supported in part by a research grant from Hyonam Laboratory Foundation, Seoul, Korea and the skillful technical assistance of Mi Ho Kim and Young Ran Kim is gratefully acknowledged.