Prognostic Factors of Patients with Thymoma
Article information
Abstract
Objectives:
To analyze the prognostic factors influencing the survival of patients with thymoma, clinical characteristics, treatment modalities and survival of patients were evaluated. The efficacy of chemotherapy was also determined.
Methods:
Retrospective study was done on one hundred patients whose diagnosis was confirmed pathologically at Seoul National University Hospital from 1981 to 1994. The staging was carried out according to the Masaoka system. Survival rate was calculated by the Kaplan-Meier method and prognostic factors were analyzed by a multivariate analysis (Weibull model).
Results:
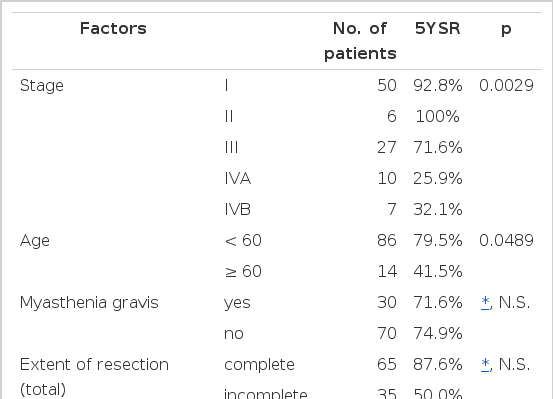
The stage of 100 patients was as follows : Stage I-50, II-6, III-27, IV A-10, IV B-7. The overall survival rates at 5 and 10 years after diagnosis were 73.1% and 58.7%, respectively. The 5-year survival differences, according to various prognostic factors, were as follows:
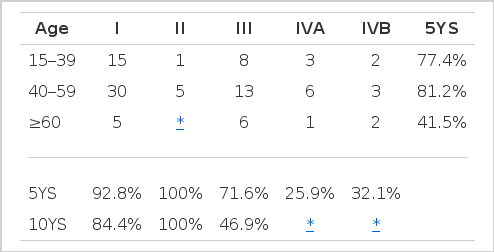
Stage : I-92.8%, II-100%, III-71.6%, IVA-25.9% and IVB-32.9% (p=0.0029).
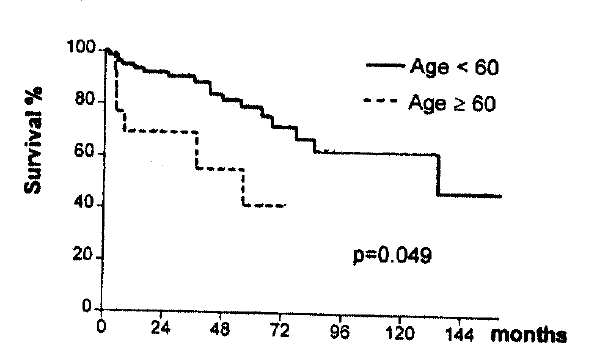
Age : <60 years-79.5% and ≥60 years-41.5% (p=0.0489).
-
Extent of resection:
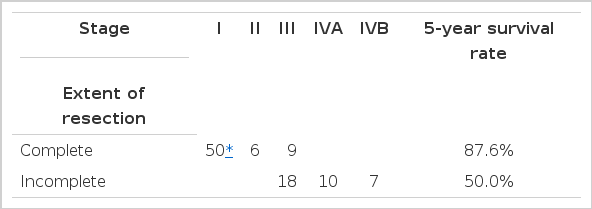
Total patients : complete reseotion-87.6% and incomplete resection-50.5% (p>0.05)
Stage III: complete resection-66.7% and incomplete reseotion-75.5% (p>0.05)
Myasthenia gravis: present-71.6% and absent-74.9% (p>0.05)
Seventeen patients were treated with a combination chemotherapy of Cyclophosphamide, Adriamycin and cisplatin (CAP). Two complete responses and seven partial responses (overall response rate of 53%) were observed with a median response duration of fourteen months. Combination chemotherapy with CAP was effective.
Conclusions:
Stage and age were the independent prognostic factors in patients with thymoma. However, the presence of myasthenia gravis or the extent of resection in stage III patients was not associated with the survival time.
INTRODUCTION
Thymoma is known as the most common tumor arising in the anterior-superior mediastinum1,2). The difficulty that investigators face is diffenentiating between benign and malignant thymoma1–5). Although some investigators suggest that histologic classifications have an influence on the survival of patients with thymoma, histologies of the two types have no distinguishable difference. Numerous papers about prognostic factors of thymoma according to clinical parameters or treatment modality have been reported. The prognostic factors in clinical parameter or treatment modality have been reported. The prognostic factors in clinical parameters are the degree of invasion (stage), age and associated autoimmune disease. It is known to be important whether complete resection is done or not6–14).
There are several Investigators who argue that complete resection played an important role in the prognosis, so they advocate aggressive surgery for complete resection in invasive thyoma. whenever possible, even in stage IVA6,7,15,16). However, the role of radiotherapy is also an important therapeutic modality for unresectable invasive thymoma6) as well as for post-operative combination therapy. Besides, several drugs have been reported to have anti-tumor activity in the thymoma17,18) and, recently, combination chemotherapy is shown to play an important role in the treatment of inoperable invasive thymoma19–22).
However, there are only a few papers written on prognostic factors in Korea23–25). This study attempted to evaluate the following items to analyse the prognostic factors influencing survival of patients with thymoma.
The clinical characteristics of thymoma patients in Korea.
The prognostic factors which are suggested above.
The significance of the complete resection in stage III patients, which some investigators advocate as the best treatment modality
The efficacy of chemotherapy (Cyclophosphamide, Adriamycin and Cisplatin) in invasive thymoma.
MATERIALS AND METHODS
Between April 1981 and July 1994, 100 patients with histologically proven thymoma were treated at the Seoul National University Hospital. Thymic carcinomas were excluded from this study. Surgical biopsies were done in 85 patients and aspiration cytologies were done in 15 patients with invasive thymoma. These fifteen patients were previously determined as inoperable by their computed tomography results. The invasiveness of each tumor was evaluated according to the clinical staging procedures of Masaoka and associates. Stage I thymomas were completely encapsulated without capsular invasion. Stage II showed microscopic or macroscopic invasion into the capsule or macroscopic invasion of mediastinal fatty tissue or of mediastinal pleura. Stage III displayed macroscopic invasion of pericardim, greater vessels or lung. Stage IVA had pleural or pericardial dissemination. Stage IVB had lymphogenous, hematogenous metastasis, or both. The degree of invasion was determined by CT in the fifteen patients who had been diagnosed with aspiration cytology Stage III, 9, Stage IVA, 4, stage IVB, 2. Stage I thymoma patients were treated by thymectomy alone, Stage II-thymectomy with or without postoperative radiotherapy, stage III-themectomy and postoperative radiotherapy with or without chemotherapy, Stage IVA-radiotherapy and/or chemotherapy, stage IVB-chemotherapy with or without radiotherapy. CAP chemotherapy (cyclophosphamide 700 /m2, doxorubicin 50mg/m2, and cisplatin 50/mg2: intravenous infusion on day 1) was administered to the eligible patients. Treatment cycles were repeated every 21 days. Patients were evaluated for response after two cycles of therapy. For patients with responsive or stable disease, treatment was continued unless grade 4 toxicity or tumor progression was noted. When complete remission was achieved, the chemotherapy was finished after two more cycles. Survival rates were computed by the Kaplan-Meier method and statistical differences were tested by a log-rank method. Multivariate analysis of prognostic factors was done using the Weibull model. A probability value, less than 5%, was regarded as significant.
RESULTS
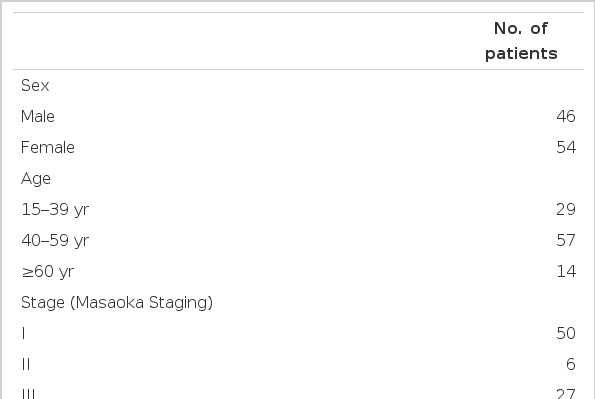
1. Clinical Manifestation
The characteristics of 100 evaluable patients are shown in Table 1. More females than males were present in this series (54% versus 46%). especially in the myasthenia gravis group (19 versus 11). The median age was 46 and there were over 50% of patients between 41 and 60 years of age. The patient population in their respective stage and age groups are displayed Table 1. The presenting symptoms of the disease are summarized in Table 2. The patients complained of abnormal finding on their chest x-ray, thoracic symptom and symptoms of myasthenia gravis. There were slightly fewer patients suffering from myasthenia gravis symptom than found in previous reports7,18,19,26,27). Myasthenia gravis developed during the follow-up or treatment period in five patients. Four of 30 patients with myasthenia gravis died due to myasthenic cirsis: Three of them had remnant thymoma: one had a complete resection of well encapsulated thymoma (stage I) and no evidence of recurrence, but the myasthenia gravis was not controlled the patient died.
2. Overall Survival and Survival by Stage
The overall survival curve shows that 73.1% of the patients are alive after five years and 58.7% after ten years in Fig. 1. Our results confirmed the validity of Masaoka and associates’ classification. Encapsulated thymoma (Stage I) was a good prognostic factor. Three out of 50 Stage I thymoma patients died. These patients died due to a traffic accident. CVA and myasthenia gravis, respectively. Thymomas with capsular or pleural invasion (Stage II) showed the best results, but 5 patients among them (total 6 patients) were followed from 3 months to 5 years. This result may be caused by a small population and short follow-up period. An intra-thoracic dissemination (stage IVA) and distant metastasis (Stage IVB) predicted a bad outcome. The difference of survival according to stage was significant (p = 0.0029) (Fig. 2) (Table 3).

Overall survival according to stage. Stage I shows a good prognosis and stage IVA and IVB shows bad outcomes. There are no survival differences between stage I and II and between stage IVA and IVB, but the result between stage I and II may have been caused by a small population and short follow-up period of stage II group.
3. Survival by Age
The patients were divided into three age groups : under 40 years, 40 to 59 years and 60 and more. Their respective survival rates were calculated and written in Table 3. The group ranging from 40 to 59 years had the best prognosis. The group older than 60 showed the worst prognosis. The difference between the former two groups is not statistically significant (p>0.05). The difference survival rate between the 40 to 59 group and the greater than 60 group was distinct and statistically significant. This result suggests that old age (greater than 60 years) be a poor prognostic factor.
We divided the patients into two groups : less than 60 and greater than 60. Then we calculated the survival rate and found a survival difference between the two groups (p<0.05) (Fig. 3).
4. Survival by Associated Myasthenia Gravis
The association of thymoma and myasthenia gravis (MG) had been considered as a bad prognostic factor because myasthenic cirsis could be a cause of death12–14,29). Nonetheless, some investigators insisted that myasthenia gravis be a good prognostic factor, because neurological treatment was improved and MG caused the early discovery of thymoma26,30). Some other investigators reported that the distribution according to stage was significantly different between myasthenic and non-myastenic groups (The proportion of invasive thymoma is higher in non-myasthenic than in myasthenic group). We divided the patients into two groups : non-invasive thymoma (Stage I) and invasive thymoma (more advanced than Stage II). We compared the survival rate in each group according to presence or absence of MG. Myasthenia did not influence the survival rate of each group.
5. Survival by Extent of Resection
The complete resection has been regarded as an important positive prognositic factor. Considering the two surgical intervention (Complete resection and incomplete resection), the survival curve showed significant difference (p<0.01)6–9,16,28). However, when we analyzed the survival difference using multivariate analysis, we found no stastistical difference (p>0.05) (Table 4). It seems that the stage factor has influenced the survival difference. We calculated the survival rate in Stage III patients by dividing them into two groups (complete resection and incomplete resection) and found no statistical survival difference, as well (Fig. 4) (Table 5).
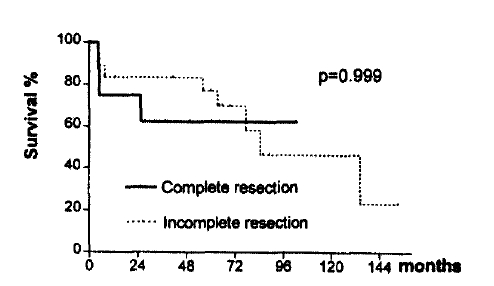
Overall survival according to extent of resection in stage III. There is no statistical difference between the complete and incomplete resection groups.
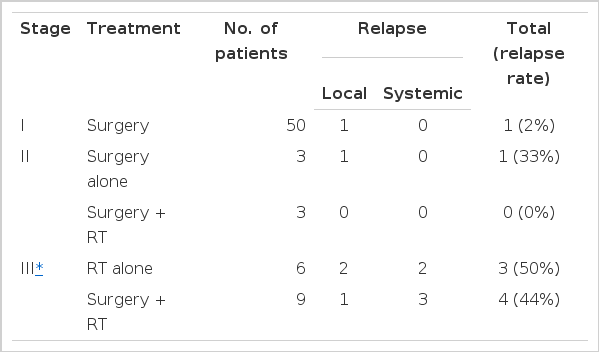
6. The Recurrence after Surgery or Radiotherapy
Tumor recurrence was observed in six patients after operation. The time lapse between operation and recurrence ranged from 9 to 84 months (median : 40 months), we analyzed tumor recurrence according to the stage. Only one (2%) out of 50 stage I patients showed relapse 84 months after surgery. Invasive thymomas recurred more frequently (1 of 6, 17% in Stage II: 4 of 9, 44% in Stage III). As the stage of disease increased, the time interval between operation and relapse decreased. Of the patients in Stage II, three patients had adjuvant radiotherapy and no recurrence. 13 patients in Stage III. who had surgical biopsy only or aspiration cytology, received radiotherapy as first-line treatment. Ten were evaluable and six complete responses (CR) and three partial responses were observed. Three patients of CR had recurrences after radiotherapy. The time lapse between the radiotherapy and the recurrence in the CR group ranged from 3 to 28 months (median : 19 months). Table 6 displays the pattern of recurrences. It seem that as the stage of the disease increases, the relapse rate after complete remission increases and systemic relapses occur more frequently than local ones.
7. The Results of Chemotherapy
Seventeen patients were treated with combination chemotherapy (Cyclophosphamide, Adriamycin and Cisplatin). There were two complete responses and seven partial responses (overall response rate of 53%) and median duration of response was 14 months (range : 2 – 38 months). Of two patients with complete response, one relapsed at mediastinum with myasthenia gravis symptom after 36 months and died 42 months later. The other one lives without evidence of disease for more than 18 months. The toxicities were tolerable.
DISCUSSION
Neoplasms of the thymus differ in histogenesis. Epithelial, lymphoid, neuroendocrine, germ cell, and mesenchymal tumors have been descrived2,31–34). According to the definiton given by Rosai and Levines,2,5) thymomas are tumors arising from the thymic epithelial cell. The traditional histological grading based on the lymphocyte/epithelial cell ratio (predominantly lymphocytic, predominantly epithelial or mixed) has become insufficient because this classification has little prognostic value4,28,36–37). In 1985, Marino and Muller-Hermelink proposed a new classification system the different subset of thymic epithelial cells was suggested38,39). Cortical thymomas were related morphologically and phenotypically to thymic cortical epithelial cells and medullary thymomas were related to medullary epithelial cells. Mixed thymomas were also discribed and further divided into three subgroups : the mixed common type, mixed with cortical predominance, mixed with eddullary predominance and sometimes arbitrary. In some series, the survival data showed signifiant differences in prognosis among three main types40–42). The medullary thymoma is comparatively rare, benign tumor, arising late in life and usually not associated with MG40). Park et al. showed no statistical correlation of invasiveness among the three types. Three cases showed that the medullary thymoma were all non-invasive; these cases were, however, too small to compare statistical significance24).
The thymoma represents seventy-five percent of the primary tumors in the anterior mediastinum and it is one of the most frequently found neoplasms in this compartment1). The patients suffering from thymoma are usually between 40 and 60 years of age, and there is no sex predilection. The chief complaints in patients with thymoma were as follows : abnormal findings on the chest X-ray without symptoms, thoracic symptoms, symptoms of myasthenia gravis etc. We found similar clinical features.
The rapid increase in the number of the patients with thymomas seen after 1974 is likely due to the radical change in the treatment policy (removal of thymus) of myasthenia gravis. This treatment policy may have caused the increased discovery of smaller-sized thymomas that would not have been detected in other ways. In this study, 50% of the thymoma patients were in Stage I. The incidence of myasthenia gravis in patients with thymoma has ranged from 7 to 54% in various series, with a mean of approximately 35%43).
Thymomas are found in one tenth of the patients with myasthenia gravis7,11,43, 44) A few cases of MG developing after operation are described. We observed five cases in which MG developed after treatment. The presence of residual thymic tissue locally or in extramediastinal sites, or the relapse of the tumor, have been suggested to explain re-explored because of newly diagnosed MG (two cases) or significant worsening of neurological symptoms (one case), had no evidence of disease at operation7). In our series, it was speculated that myasthenic symptoms were associated with the presence of thymic tissue because 5 patients, who were newly diagnosed as having MG, had residual disease or relapse. Even though MG may have been the cause of death, the presence of MG was not a poor prognostic factor in thymoma patients in our study. This conflicted with the reports of the 1960s and early 1970s. Bernatz14) ovserved that MG did not affect survival if the thymomas were invasive but survival was decreased if the tumors were non-invasive. However, some studies reported a higher survival rate in MG pateints14,32,48,47. They explained that the earlier diagnosis of thymoma (permitted by the development of MG symptoms and the improvement in treatment of MG) might account for this finding. We calculated the difference of the survival rate in invasive and non-invasive group separately according to the presence or absence of MG, but found no survival difference.
About 40% of the patients had thoracic symptoms and the remainder of such tumors are usually detected on routine chest radiography11). Paraneoplastic phenomena, other than MG, occurred with thymoma in about 10% of the patients11,48,49). These disorders largely consisted of autoimmune-related syndromes or hematologic abnormalities. Thymoma-associated systemic syndromes, other than MG, were ovserved in 2 cases. One case was pure red cell aplasia and the other was systemic lupus erythematosus. Pure red cell aplasia are found in about 5% of patients with thymomas, and thymectomy induced remissions of the disease in 38% of the patients. However, our case did not show remission, even after thymectomy1).
The classification of Masaoka and associates based on the extent of tumor invasion is valuable. Survival curves show that locally invasive thymomas have a less favorable outcome compared to encapsulated forms. When intrathoracic disseminations or distant metastasis are found, survival rates decrease significantly. Even though we found no survival difference between intrathoracic dissemination (Stage IVA) and metastasis (Stage IVB), staging was found to be the most important prognostic factor in our study. Wilkins50) argued that patients with pathologic Stage I thymoma, although they merit careful follow-up, need no adjuvant therapy and have an excellent prognosis. For stage II thymoma, it is generally agreed that the best treatment is an operation, followed by radiotherapy21). We agree with this treatment strategy, even though the results of our study were too small to draw definitive conclusion. For stage III, radical operation followed by radiotherapy is considered the treatment of choice21). Several investigators argue that complete resection play an important role in the prognosis and they advocate aggressive surgery (complete resection) in invasive thymoma, even in stage IVA6,7) But we found no stastistical difference in the group of thymoma patients in stage III, whether complete resection was done or not. Instead, we found early death and high relapse rates (44%). Radiotherapy was recommended in unoperable cases, but even when patients had a complete remission, half of them relapsed. In Stage III, we were faced with the need of systemic therapy because the systemic rather than local relapse was more common. This need occurred even though patients had complete remission by local modality treatment (complete resection and post-operative RT or RT alone). At present, combination chemotherapy is used after incomplete resection or after incompleter resection and radiotherapy. The overall response rate to combination chemotherapy was about 50% and complete response was about 10–40%21,22,51,52). Our study showed similar results. In this study, the patients, who had relapsed after local modality treatment, had systemic CAP chemotherapy. Radical resection was difficult in some cases due to locally invasive lesion. In these cases, some authors have suggested that neoadjuvant chemotherapy followed by surgery may be useful to reduce the size and degree of the infiltration of the lesion, thus increasing the probability of radical operation21). This therapy may be helpful to pervent systemic relapses, but there is no Phase III randomized controlled study.