Combined Cis-platinum and Alpha Interferon Therapy of Advanced Hepatocellular Carcinoma
Article information
Abstract
To evaluate the clinical efficacy of α-inferferon(IFN-α) plus cis-platinum in hepatocellular carcinoma (HCC), 56 inoperable patients with HCC were divided into IFN-α plus cis-platinum treated group (n=30) and no antitumor therapy group (n=26). The survival of IFN-α plus cis-platinum treated patients was significantly better than that of patients who received no antitumor therapy (p=0.001). Median survival time was 33 weeks and 14.0 weeks, respectively. The cumulative estimated survival rates of our IFN-α plus cis-platinum treated group (93.5% at 3mo. 75.0% at 6mo) were for longer than that of the no antitumor therapy group (84.6% at 3mo, 57.7% at 6mo). Objective tumor regression, greater than 50%. was observed in 13.3% (4 of 30) of patients receiving IFN-α plus cis-platinum. By the univariate analysis, the absence of portal vein thrombus (p<0.05). alkaline phosphatase lesser than 280 U/L (p=0.001), total bilirubin less than 2.0 mg% (p<0.05). serum triglyceride less than 155 mg/dl (p<0.05) were shown to be the factors most significantly favoring a better survival.
By the multivariate analysis, using Cox proportional hazards model, IFN-α plus cis-platinum treated group (p=0.0001). alkaline phosphatase less than 280 mg/dl (p=0.005, the absence of portal vein thrombus (p=0.020) were independent favorable prognostic factors. We conclude that IFN-α plus cis-platinum is useful in patients with inoperable HCC and the above favorable prognostic factors may also be useful in the design and analysis of future clinical trials of systemic chemotherapy for HCC
INTRODUCTION
Hepatocellular carcinoma (HCC) is a major cause of cancer death in many Asian countries1,2). Since almost all patients have extensive intrahepatic spread of the tumor and/or metastatic disease at the time of diagnosis, the disease is usually unresctable. Although some of these patients were treatable surgically, the disease is actually too extensive to perform surgical attempt or which will recur after resection. Therefore, they might be considered candidates for systemic or regional therapy. Until now, several reports for systemic chemotherapy of HCC had shown an unsatisfactory response of less than 10%3,4). Therefore, the development of new active anticancer agents is essential, Cis-platinum has a broad spectrum of antineoplastic action and there has been many reports demonstrating favorable effects for the treatment of various malignant diseases5,6). Interferon has been shown to have a powerful antiproliferative effect on the human hepatoma cell line7, 8). In this study, we report our experience of IFN-α plus cis-platinum treatment in 30 patients with HCC. The current study was also undertaken to evaluate the survival time of patients with HCC and find prognostic factors which allow the selection of patients with a life expectancy long enough to undergo therapy.
MATERIALS AND METHODS
1. Patients
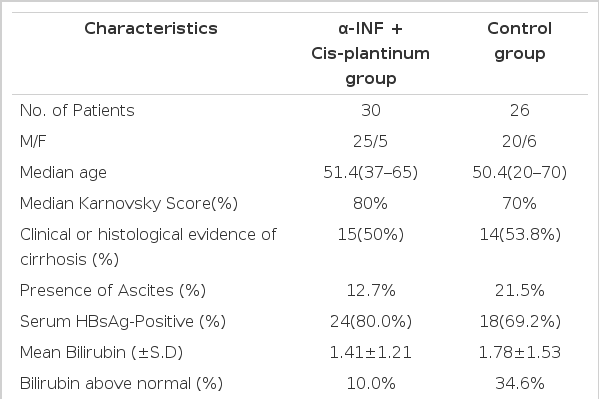
Between April 1992 and July 1994, at the Department of Internal Medicine. Kosin Medical College, Pusan, Korea, 56 patients with HCC were entered on this study. Their tumor was inoperable because of underlying severe cirrhosis or extrahepatic metastasis or poor general condition. The diagnosis of HCC was made histologically in 32 patients and in the remaining 24 patients diagnosis was based on markedly elevated alphafetoprotein (AFP) values (>500ng/ml, range 1271–32,000 ng/ml) with space occupying lesions demonstrable by ultrasonography or computer tomographic scan. Twenty-nine were cirrhotics. The diagnosis of underlying cirrhosis was based on clinical and laboratory features plus endoscopic evidence of esophageal varices and positive liver scan. The 56 patients were divided into no antitumor therapy group (26 cases, 42%) and treatment group (30 cases, 58%) and are summarized in Table 1. Karnovsky score was greater than 70% (i.e., the patient was ambulant and able to take care of most daily needs). Forty-five patients (80.4%) were men (mean age, 63 years, range 37–65 years) and eleven (19.6%) patients were women (mean age, 64 years, range, 46–61 years).
Markers of previous or present HBV infection were tested by radioimmunoassay (RIA) (Abott laboratories Kit), and 42 of the 56 patients (75%) were HBsAg positive. Although the control group had more patients with bilirubin level greater than 2.0mg%, no statistical difference was seen between the two groups with respect to any of the other parameters. Patients were not enrolled if they had received any prior chemotherapy or immunotherapy.
2. Trial Protocol
We performed a retrospective study of IFN-α plus cis-platinum treated group (n=30) and no antitumor therapy group (n=26).
Cis-platinum was given by slow intravenous infusion in a dose of 60 mg/m2 diluted with 5% dextrose at 4 weekly interval9). Anti-emetic agents were given prophylatically and a daily fluid intake of at least 3L was ensured. Interferon was administered on alternative day and doses of 3×106 units intramuscularly for 3 months consecutively10).
In all patients, serum urea nitrogen and serum creatinine levels were closely monitored to check renal toxicity before each treatment course, and treatment would be withheld if serum creatinine value, which was normal before treatment, had increased above 2.0mg%.
Treatment would be also withheld if performance status was below 50% and there was evidence of severe myelosuppression : white cell count below 2000/mm3 and/or platelet count below 25,000/mm3.
The dosage of IFN-α was reduced by one half if any severe untoward events developed, or significant leukopenia (<2000/mm3) and thrombocytopenia (<50,000/mm3). Therapy was restarted when patients recovered.
3. Prognostic Factor Analyzed
Pre-treatment clinical and laboratory variables were investigated for their relationship to long-term survival. Each variable was divided into two sub-groups. The variables included the following : age less than 60 yr or more than 60 yr : Sex; HBsAg Status or absence of cirrhosis ; serum albumin less than 3.5 mg/dl or more than or equal to 3.5 mg/dl: serum total bilirubin less than 2.0 mg/dl or more than or equal to 2.0 mg/dl; alkaline phosphatase less than 280 U/L or more than or equal to 280 U/L : AST/ALT ratio less than 2.0 or more than or equal to 2.0 ; serum triglyceride less than 155 mg/dl or more than or equal to 155 mg/dl: serum AFP less than 500 ng/ml or more than or equal to 500 ng/ml : extrahepatic metastases present or absent : presence or absence of portal vein thrombus : presence or absence of ascites : response to therapy (partial response, stable, progression).
4. Criteria for Survival Time and Tumor Regression
Survival time was defined as the time between the day of diagnosis and the time of the patient’s death or the time of last follow-up observation. Predefined criteria of response to treatment were as follows11) : complete remission indicated disappearance of all known disease of HCC : partial remission : a≥50% reduction in the product of the two largest perpendicular diameters in patients with a single echographic tumor lesion. Stable disease was defined as lesion, unchanged for 8 weeks. In fact, in less than 1 25% decrease or less than a 25% increase in the size of measurable lesions, progressive disease was defined as a greater than or equal to a 25% increase in the size of measurable lesions or the presence of new areas of malignant disease. Tumor size was estimated by ultrasonography at 4 week intervals and by CT at 12 week intervals.
5. Statistical Methods
The χ2 test was used to test for differences in the response rate. Survival curves were calculated by the Kaplan-Meier method12) and statistical significance was evaluated by log-rank test12). Multivariate regression analysis was conducted to identify the independent prognostic importance of each variable study. The variables entered into the Cox proportional hazard model13) included age. sex and those found to be associated with survival based on the log-rank test. Statistical significance was defined as a p value of 0.05 or less.
RESULTS
1. Survival
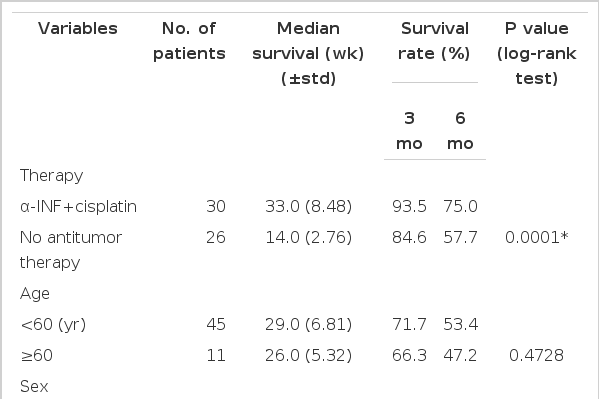
Of the 56 patients studied, 14 (25.0%) were still alive. As analyzed by the Kaplan-Meier the method, the survival curve for patients is shown in Fig. 1. the IFN-α plus cis-platinum treated patients (n=30) had significantly better survival than did the no antitumor therapy group (n=26. p=0.001 : by Log-rank test). The median survival for the IFN-α and cis-platinum treated patients was 33.0 weeks, that for the patients receiving no antitumor therapy was 14.0 weeks. Cumulative survival rate in the treated group with IFN-α plus cis-platinum was 93.5% at 3 months and 75.0%, 23.5% at 6 months, 1 year, respectively. In the no antitumor therapy group, cumulative survival rate was 84.6%, 57.7%, 7.2% at 3 months, 6 months and 1 year, respectively.
2. Response to Treatment
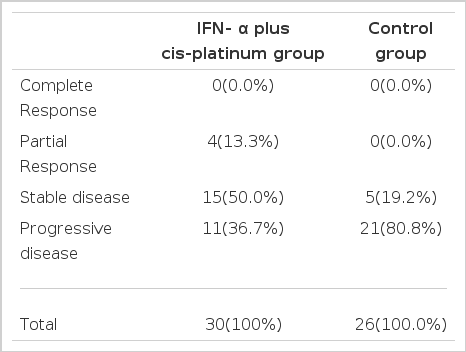
Table 2. showed the comparative results of HCC between IFN-α plus cis-platinum treated group (n=30) and the no antitumor therapy group (n=26). Among our 30 patients, with IFN-α plus cis-platinum treatment, only 4 patients (13.3%) had a partial response lasting 3 months. These patients had survived for 18 months after the onset of therapy and had been treated at the 9th chemotherapy. Fifteen patients (50.0%) remained patients (36.7%) had disease progression at this time, with a median interval of 3 months to antitumor therapy who survived for more than 3 months, showed objective tumor regression greater than 50%, including 5 patients (19.2%) with stable disease and 21 patients (80.2%) with progression disease. IFN-α plus cis-platinum treatment was still associated with fewer patients with progressive or stable tumor than it was with patients receiving no antitumor therapy (Table 2). We found no objective evidence of correlation between tumor regression and survival (p>0.05).
3. Univariate Analysis
The difference in survival among the various subgroups with each prognostic factors were evaluated using log-rank tests. Median survival and 3, 6 months survival rates for each prognostic factor are shown in Table 3. The absence of portal vein thrombus (p<0.05). serum alkaline phosphatase less than 280 U/L (p=0.001), serum total bilirubin less than 2.0 mg/dl (p<0.05), serum triglyceride less than 155 mg/dl (p<0.05) were the factors significantly favoring a longer survival (Fig. 2).
Univariate Analysis of Prognostic Factors Associated with Survival in Patients with Unresectable HCC

A : Survival rates in relation to serum total bilirubin(TB) : TB<2.0mg% versus ≥2.0mg% : p<0.05 B : Survival rates in relation to serum Alkaline-phosphatase(ALP) : ALP180-280 μ/L versus ALP>280 μ/L C: Survival rates in relation to portal vein thrombosis(PVT) : PVT(+) versus PVT(−) : P<0.05 D : Survival rates in relation to Triglyceride(TG) : TG 50-155mg/dl versus TG>155mg/dl: p<0.05:
4. Multivariate Analysis
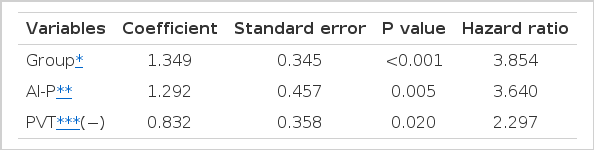
Group with the IFN-α and cis-platinum treated patients (p=0.0001), Serum alkaline phosphatase less than 280 U/L (p=0.005), the absence of portal vein thrombus (p=0.020) was significantly related to survival according to this analysis (Table 4). However HBsAg status, tumor size, serum AFP and albumin levels and total bilirubin levels and ascites had no significant influence on survival in the final multivariate model.
5. Side Effects
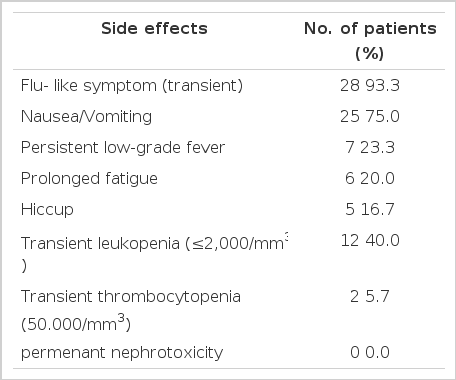
The toxicity was, as might be anticipated, for an INF-α and cis-platinum combination. Almost all patients (28 of 30, 93.3%) receiving IFN-α therapy experienced a flu-like illness during the first 3 times of injection, but the flu-like symptom were RELIEVED by oral acetaminophen. The nadir of white blood cell counts was observed in 12 patients (40.0%) at 2 weeks after the beginning of treatment and the lowest leukocyte count recorded was 600/mm3, but these counts recovered their initial levels within 2 more weeks. Thrombocytopenia was less frequent and less severe, 2 of 4 patients achieving partial response had a platelet nadir less than 50,000/mm3, and none had a nadir less than 25,000/mm3. Cell counts returned to baseline values within two weeks after completion of therapy. Seven patients (23.3%) had persistent low grade fever (37 °C–38.2 °C). Nausea, vomiting was the most frequent side effect, and was observed in 25 patients (75.0%), usually starting within 3 hours after treatment and persisting for a few additional days. Most patients relatively tolerated it and did not necessitate discontinuation of therapy. Serum urea nitrogen levels and serum creatinine levels were closely monitored to check renal toxicity. None of our patients had biochemical evidence of nephrotoxicity. Hiccups occurred for a few days after treatment in 5 patients (16.7%). (Table 5)
DISCUSSION
Of the 30 patients treated with IFN-α plus cis-platinum, 4 patients (13.3%) showed partial response, the median survival time and cumulative survival rate at 3 months and 6 months were 33.0 weeks and 93.5%, 75.0%, respectively. When compared with IFN-α plus cis-platinum group, no antitumor therapy group showed that the median survival time and cumulative survival rates were 14.0 weeks and 84.6%, 57.7% at 3, 6 months respectively. This study demonstrated that IFN-α plus cis-platinum was of value in prolongation survival and inducing tumor regression in patients with inoperable HCC. In an earlier study of the natural history of patients with inoperable HCC, the median survival in the group receiving no antitumor therapy was only 3.5 weeks14). The longer survival of the group receiving no antitumor therapy in this study is most likely attributed to good Karnovsky score status above 70%. But the median lengths of survival of Okuda15) and Attali16) in untreated patients with HCC were 16.0 weeks and 11.0 weeks, respectively, which were not different from that of this study. Although this study was made retrospectively, these results made it possible to evaluate the clinical effect of IFN-α plus cis-platinum and the selection of patients was considered to be unbiased.
Until recently, though the treatment of HCC remained still unsatisfactory. Adriamycin has been the most frequently administered drug for treatment of HCC among various chemotherapeutic modalities17, 18). Several reports demonstrated the response rates of Adiramycin to HCC have varied over a wide range from 15–34%19,20). It seemed that the comparison of variable results was difficult because dosage and definition of response differed widely. Recently. Johnson20) reported that Adriamycin induced tumor regression was 3.3%–10% in patients with HCC and similar results were reported in many studies21, 22). But, there have been many reports concerning the high incidence of fatal side effects, such as cardiotoxicity23, 24). Therefore. alternative therapy for treatment of HCC is essentially needed. Interferon has been shown to have a powerful antitumor effect, correlated with its antiproliferative against a human hepatoma cell line in vivo and vitro7), and immunomodulatory properties8). In clinical study, IFN-α induced tumor regression of 7–41% was observed25). Cis-platinum has a broad spectrum of antineoplastic action correlated with inhibition of DNA synthesis26) and there has been several reports demonstrating favorable effects5). The response rate of cis-platinum to HCC was 8.8–15.4%6). Compared with previous trials, showing Adriamycin plus IFN-α therapy induced tumor regression of 50% in only 3.3% of patients with HCC27), a 13.3% objective regression rate in this study, anticipating synergistic effect for IFN-α plus cis-platinum combination. was shown to be superior to Adiramycin plus IFN-α therapy. In fact, the response rate of Adriamycin plus IFN-α was actually less than what would be anticipated from reported experiences using either drugs alone. In context, Adriamycin plus IFN-α therapy seems that there was no synergistic interaction between two drugs for treatment of HCC. But IFN-α and cis-platinum therapy might seems that there is synergistic effect. The reason for the difference in the response rate, median survival time and survival rate between IFN-α plus cis-platinum and previous several trials was unknown. Several possible explanations exist. First, the number of patients evaluated in the previous study, involving Adriamycin plus IFN-α therapy, was relatively small compared with those in this study. Second, there may be an ethnic and/or regional difference in the biological characteristics of HCC itself in the tumor responsiveness to therapy. Previous studies in North American28) or Chinese patients24, 25) showed no benefit or minimal beneficial effect of IFN-α or cis-platinum. Third, the longer survival rates and median survival time in this study, we would like to suggest three points. First, it was mainly in the treated group that significant prolongation of survival was observed. Second, despite the terminal progression of tumors, four patients of these treated group showed significant tumor shrinkage compared with the radiologic dimensions of the tumors before therapy. Third, although we observed that IFN-α plus cis-platinum increased the survival of patients with HCC, we found no objective evidence of correlation between tumor regression and survival. However, despite the absence of a benefit between tumor regression and survival, it should be noted that the IFN-α plus cis-platinum achieves partial response in a few patients and this prompts slower tumor progression, which may delay the appearance of tumor-related symptoms. Our observation also suggests that survival is probably more related to the advanced status of the disease of these patients on entry into the study than to progression of the tumor.
This study was also carried out to evaluate the factors affecting survival, thus enabling us to confirm important prognostic factors, predict the long them outcome and plan future clinical trials of the therapy of HCC. In our univariate analysis, 4 of 12 variables tested had a significant prognostic value, such as the absence of portal vein thrombus, alkaline phosphatase less than 280 U/L, total bilirubin less than 2.0 mg/dl and serum triglyceride less than 155 mg/dl showing significantly longer survival using log-rank tests.
Age, sex, tumor size, metastasis, presence or absence of cirrhosis, serum albumin more than or equal to 3.5 gm/dl, no ascites and serum AFP less than 400 ng/ml had no significant effect on survival. Recently, Sutton and co-workers29) reported that age, sex, ascites and cirrhosis were found to have a prognostic value in their analysis. In our study, neither ascites nor cirrhosis affect the survival of HCC. This is unbelievable because ascites and cirrhosis in patients with HCC were usually considered poor prognostic variables30). The reason why ascites or cirrhosis did not predict survival remains obscure. The absence of the prognostic value of ascites or cirrhosis might be due to the small number of cirrhosis or the late stage of HCC in this study. AFP did not appear in this study to be a parameter significantly related to survival. The prognostic value of AFP levels has already been debated in the past with different conclusion31–33). A close relation between AFP levels and the tumor burden has been denied by Cotton32) and Ebara31), whereas Sheu31) found a significant correlation between the progressive rise of AFP and the increase of the neoplastic mass. In this study, we confirmed that there was no statistical correlation between AFP and tumor progression.
Our multivariate analysis showed that only three factors, the treated group with IFN-α plus cis-platinum, portal vein thrombus and alkaline phosphatase had an independent prognostic variable to survival. Chlebowski28) reported that serum bilirubin affects prognosis of HCC. Calvet34) also reported that serum bilirubin was a predictor of survival. It is generally agreed that elevated serum bilirubin level has been shown to adversely influence th survival of patients with HCC. regardless of treatment. In this study, serum bilirubin was not found to be significant in multivariate analysis. However, bilirubin was found to be significant in the univariate analysis. Although not all the variates identified as significantly related to survival, using univariate analysis, this discrepancy may have occurred because the influence of the individual factor on each other was not in account for the univariate analysis but was in the multivariate analysis.
Hypercholesterolemia is an important paraneoplastic manifestation in patients with HCC35). The incidence of Hypercholesterolaemia in HCC patients has been reported as varying from 11 to 38%36).
According to Hwang37), who reported that hypercholesterolemia or hypertriglyceridemia HCC patients tended to have large tumor volume, none of the small HCC patients had high cholesterol or triglyceride levels, and serum triglyceride levels in HCC patients fell to the normal range after surgical resection or chemotherapy of the tumor, and rose to abnormal level again after tumor recurrence.
These findings may indicate that re-elevated serum cholesterol or triglyceride levels in HCC patients were associated with tumor recurrence and the presence of a viable tumor mass.
Although the exact mechanism of hypercholesterolemia or hypertriglyceridemia in HCC patients has not been fully understood, by the view of Hirayama38), he postulated that there may be autonomous cholesterol or triglyceride biosynthesis inn the tumor cell, followed by cholesterol or triglyceride release into the circulation, causing hypertriglyceridemia or hypercholesterolemia.
Therefore, serum cholesterol or triglyceride levels may be a clinically important prognostic significancy in therapy with IFN-α and cis-platinum.
We, thus, conclude that IFN-α and cis-platinum combination therapy may lead to higher response rates and the improvement of survival for inoperable HCC patients and above mentioned favorable prognostic factors may be also be useful in the design and analysis of future clinical trials for systemic chemotherapy of HCC.