A Case of Intraductal Papillary Tumor of Pancreas Associated with Mucinous Ductal Ectasia
Article information
Abstract
Intraductal papillary neoplasm associated with mucinous ductal ectasia is an uncommon cystic disease of the pancreas. This tumor is characterized by the multilocular cyst lined by mucin producing cells with variable degrees of atypia and ductal ectasia. In addition, this tumor has a favorable prognosis in contrast to other mucinous cystic tumor of the pancreas. Reports on intraductal mucin producing tumor are quite limited and most of them are found in Japanese literature.
We report a case of intraductal papillary adenocarcinoma with marked dilatation of the pancreatic duct filled with mucin in a 72-year-old female patient.
INTRODUCTION
In 1980, Ohashi et al. described a special type of pancreatic cancer characterized by dilatation of the main pancreatic duct with intraluminal papillary projecting tumor1). Since then, more than 50 similar tumors have been reported by Japanese authors and various names were given to these tumors. Intraductal mucin-producing tumor represents a distinct clinicopathological entity. This tumor shows a papillary proliferation of well-differentiated mucin secreting cells with marked dilatation of the main pancreatic duct. Recently a common classification of these neoplasms was proposed under the definition of intraductal papillary mucinous neoplasms (IPMN) according to the WHO classification of pancreatic tumor2), and this disease was also named mucinous ductal ectasia by clinicians3). In Korea, Song et al. reported a case of ductectatic type mucinous cystadenoma of the pancreas in 19924).
We experienced a case of intraductal papillary mucinous tumor of the pancreas with marked ductal ectasia in a 72-year-old woman and report this case with a review of the literature.
CASE REPORT
A 72-year-old woman was referred to our hospital for the evaluation of the pancreatic mass.
She presented with a one month history of palpable abdominal mass and body weight loss of 5kg. Also, she complained of loose stool and dyspepsia occasionally for three months. Her family and past medical history were not contributory. On admission, pulse rate was 68/min, blood pressure 140/80mmHg, respiration rate 18/min and body temperature 36.7°C. On physical examination, she appeared relatively well and the sclerae were anicteric. There was a palpable nodular non-tender mass with hard consistency in the epigastric area. The remainder of the physical examination was normal.
Laboratory studies included hemoglobin 12.1g/dl, white blood cell 3,800/mm3, platelet 201,000/mm3, total protein 6.7g/dl, albumin 3.7g/dl, AST 18U/L, ALT 15U/L, total bilirubin 0.4mg/dl, direct bilirubin 0.1mg/dl, alkaline phosphatase 119U/L, fasting blood glucose 109mg/dl, serum amylase 66IU/L, lipase 159IU/L and CA 19-9 3.0U/ml.
The abdominal computed tomography (CT) scan showed 5cm-sized protruding cystic mass in the pancreatic tail with calcification (Fig. 1). The entire main pancreatic duct and its branches were markedly dilated and pancreatic parenchyme was severely atrophied (Fig. 2). During the endoscopic retrograde pancreatography (ERP), we found patulous orifice of the papilla, and mucin was extruded through it. ERP showed diffuse dilatation of the entire pancreatic duct, but opacification of the pancreatic duct was difficult due to the presence of thick mucin (Fig. 3). Sono-guided aspiration cytology of the cystic mass in the pancreatic tail revealed numerous large sheets of tall columnar cells within the mucinous background. The cells had cytoplasmic mucin vacuoles and showed nuclear enlargement with increased nuclear cytoplasmic ratio.
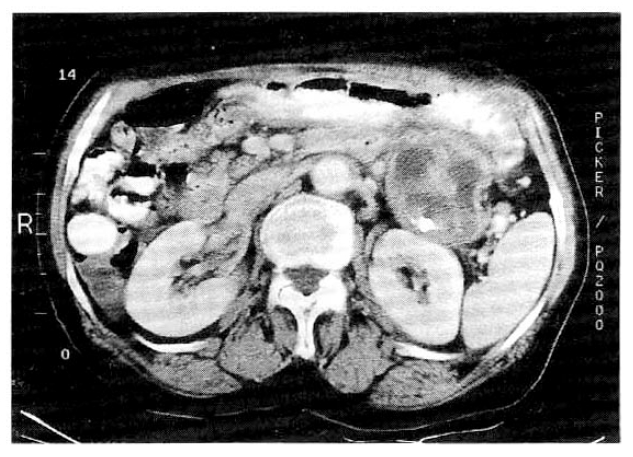
The abdominal CT scan showed 5cm-sized protruding cystic mass in the pancreatic tail with calcification.
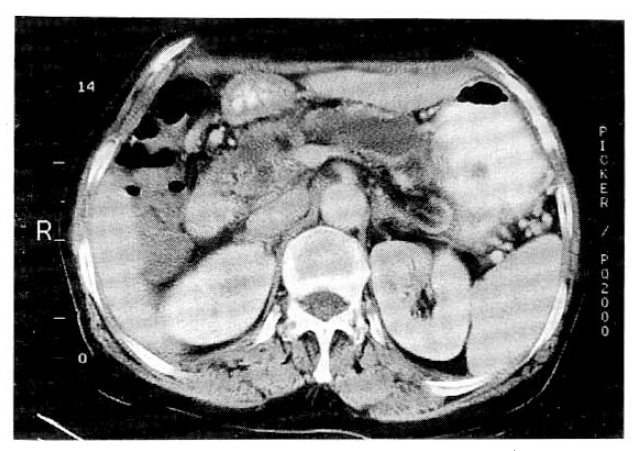
The abdominal CT revealed that the entire main and branching pancreatic ducts were markedly dilated and pancreatic parenchyme was severely atrophied.

Endoscopic retrograde pancreatogram showed poor visualization of the pancreatic duct, but diffuse dilatation of the entire pancreatic duct, with vacuolization in it, was discernible.
With the diagnosis of ductectatic type mucin producing tumor of the pancreas, exploratory laparotomy was done and resection of the body and tail of the pancreas and splenectomy were carried out. At operation, the pancreas appeared to have brownish, protruding tumorous lesion in its body and tail portion. There was no lymph node enlargement.
The resected portion of the pancreas revealed a cystic mass in the tail. The multilocular cyst was 6×5.5×4.5cm in size and connected with the branch duct. The main pancreatic duct was diffusely dilated and its maximal diameter was 2.4cm. The lumen of the pancreatic duct and cyst was filled with mucin and their walls showed diffuse intraluminal papillary protrusion (Fig. 4).

The resected portion of the pancreas revealed a cystic mass in the tail. The multilocular cyst was 6×5.5×4.5cm in size and connected with the branch duct. The entire main and branch ducts were diffusely dilated. The lumen of ducts were filled with mucin and their walls showed diffuse intraluminal papillary protrusion.
Microscopically, the ductal epithelium and cyst wall were replaced by papillary proliferation, tall columnar cells of mucinous type. The large proportion of duct and cyst showed papillary hyperplasia or adenomatous change of the epithelium and carcinomatous change in some areas (Fig. 5). There was multifocal mucin spillage with dystrophic calcification in the stroma, but definitive invasion of the stroma by tumor cells was not seen. The remaining pancreatic parenchyme revealed severe atrophy. There was no evidence of tumor metastasis in the peripancreatic lymph node. These findings were consistent with the intraductal papillary tumor with mucinous ductal ectasia.
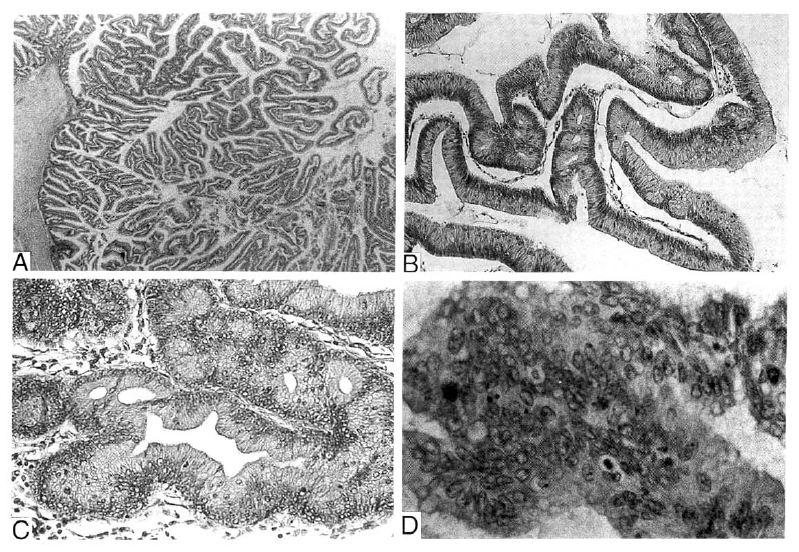
Microscopically, the ductal epithelium and cyst wall was replaced by papillary or villous proliferating tall columnar cells of mucinous type(A: H&E, ×40). The degree of proliferation and cellular atypism varied in areas. The large portion of the duct and cyst showed papillary hyperplasia(B: H&E, ×100) or adenomatous(C: H&E, ×100) change of epithelium with atypia. In some areas, marked nuclear atypism and architectural complexity with cribriform pattern and irregular budding was identified and this finding was regarded as carcinomatous change(D: H&E, ×200).
At 3 months after the operation, the patient was clinically well.
DISCUSSION
Intraductal papillary mucinous neoplasm (IPMN) of the pancreas, which comprises papillary growth of the epithelium along the duct with mucin production, is a relatively rare cystic tumor of the pancreas2). This tumor shows a papillary proliferation of the ductal epithelium with replacement of mucin producing cells exhibiting various degrees of cellular and structural atypia. It is characterized by the marked dilatation of the pancreatic duct. In IPMN, mucus produced by the tumor cells is not retained in the cyst but flows out into the main pancreatic duct which is in communication with the cyst2, 3, 5, 6, 7).
IPMN of the pancreas has become an increasingly recognized clinicopathological entity because of characteristic pancreatograms and favorable prognosis after resection. This tumor is distinguished from ordinary mucinous cystic neoplasms in many respects. Most patients are men over 60 years old, who presented with abdominal pain and had a long history of chronic pancreatitis2, 6). The tumor is mainly located in the head of the pancreas2, 3, 5, 8). In contrast, mucinous cystic neoplasm occurs mainly in young women and it occurs predominantly in the tail of the pancreas5, 9). Generally a favorable prognosis is expected for IPMN, even in malignant cases, because it is a slowly growing tumor and metastatic spread is rare2, 6).
Because these IPMNs eventually cause obstruction of the main pancreatic duct, they cause symptoms of chronic pancreatitis that may be present several years before the diagnosis. If the tumor involves the ampulla, jaundice may occur6).
At the abdominal ultrasound (US) and CT findings, it is necessary to differentiate the serous cystadenoma, mucinous cystadenoma or pancreatic malignant tumor. At CT, IPMN of the main duct shows either diffuse or segmental dilatation of the pancreatic duct and is associated with parenchymal atrophy. It is sometimes possible to identify intraductal calcification. The mucinous tumor arising from the branch ducts is characterized by cystic ectasia of the ducts and forming a cystic mass with lobulating contour that involves, usually, uncinate process10). In our patient, the main tumor mass was located in the tail of the pancreas and diffuse dilatation of the main and branch ducts was noted. Occurrence of the tumor of the branch duct in sites other than the uncinate process is very rare. In this case, the symptom of chronic obstructive pancreatitis was not remarkable probablydue due to the tumor location.
Endoscopic retrograde pancreatogram (ERP) is most useful for establishing the diagnosis of IPMN3, 10). During ERP, the intraduodenal protrusion of the papilla as well as mucin leaking from the dilated papillary orifice or fistulous tract2, 8, 11) can be observed. Opacification of the duct is often difficult due to the presence of mucin and the patency of the papillary orifice. Opacification of the duct shows its diffuse or segmental dilatation and some filling defects because of the presence of mucin.
Different histologic patterns, from hyperplasia, adenoma or dysplasia to malignant transformation, may frequently coexist2). Therefore diagnosis of benignity on the basis of transcutaneous aspiration is unreliable. Surgical biopsy should be necessary for the conclusive diagnosis. Several investigators have reported the concurrent presence of hyperplastic or adenomatous columnar epithelium adjacent to the malignant epithelium. So, the sequence of pancreatic ductal hyperplasia-adenoma-carcinoma has been suggested. As the lesions increase in size, the degree of epithelial atypia becomes higher, resulting in invasive carcinoma2, 5). Nagai et al. reported that the mean size of the hyperplasia, adenoma and adenocarcinoma were 2.0cm, 3.0cm and 4.8cm in diameter, respectively2). The tumor in our case was more than 5cm in diameter and showed coexistence of papillary hyperplasia, adnoma and carcinoma, histologically.
The diagnostic delay may lead to the great growth of the tumor with malignant transformation. In malignant cases, a radical operation should be performed. Moreover, even in benign cases, tumor resection should be considered because of the malignant potential of IPMN2, 5). Early diagnosis with imaging modality, CT and ERP, allow surgical resection as a definite treatment. In the carcinoma of IPMN, infiltration of the peripancreatic tissue and metastasis to the lymph node develop late in the course of the disease, compared with the rapid growth of pancreatic duct adenocarcinoma6). Thus their biological behavior is much less aggressive than that of the invasive ductal carcinoma. The information of the long-term outcome of this tumor is limited at present.
In summary, IPMN is a rare cystic disease of the pancreas characterized by low malignancy. This case shows unusal location of intraductal papillary mucinous tumor with marked ectasia of the pancreatic duct and the various histologic findings.