HLA and Insulin-Dependent Diabetes Mellitus in Koreans†
Article information
Abstract
Specific allelic associations vary among ethnic groups. We studied the distribution of HLA-A, -B, -C and -DR antigens in 41 patients with insulin-dependent diabetes mellitus (IDDM) and 280 unaffected persons in Korea. HLA typing was performed by the standard microlymphocytotoxicity test using antisera supplied by the Third Asia-Oceania Histocompatibility Workshop Conference (3rd AOHWC, 1986).
There was no association between HLA-A, -B, or -C and IDDM. However, the frequencies of HLA-DR3 and HLA-DR4 were increased in the patients as compared with the controls (19.5% vs 4.3%, RR 5.4, corrected p < 0.005 for DR3 and 61.0% vs 36.4%, RR 2.7, corrected p < 0.05 for DR4). Also a decreased frequency of HLA-DR2 was found in the patients with IDDM (9.8% vs 32.1%, RR 0.3, corrected p < 0.05).
These results emphasize the differences in HLA-IDDM associations among different ethnic groups.
INTRODUCTION
Many studies of different ethnic groups have conclusively shown that the genetic susceptibility to insulin-dependent diabetes mellitus (IDDM) is HLA linked1). Among the various HLA specificities, the D/DR locus antigens have the closest associatioin with this disorder, the deviation in the frequency at the other HLA loci being secondary to their linkage with these antigens2).
The most extensively studied are the European and North American Caucasoid populations, in whom DR3 (and B8) or DR4 (and B15) increases the risk of developing IDDM, while DR2 (and B7) exerts a protective influence3). The specific allelic association, however, varies among ethnic groups, such as DR4 and DRw9 restricted to the Japanese population or DR3 and DRw9 restricted to the Chinese population4).
Since there have been limited data on the HLA associations in Korean patients with IDDM5–7), we determined whether Korean IDDM patients have the same or a higher incidence of certain HLA antigens as Caucasians, Japanese and Chinese or possibly other HLA antigens in the present study.
SUBJECTS AND METHODS
Forty-one unrelated Korean patients with IDDM were studied, 16 males and 25 females whose ages at onset ranged from 6 to 40 yr (average 24 yr). Clinical criteria for the selection of patients included onset before 40 yr; body mass index less than 25; ketosis-prone disease, and insulin dependence to control symptoms and to prevent basal ketosis. None of patients had other clinically detectable endocrinopathies or autoimmune disorders. Two hundred eighty healthy Korean volunteers served as controls. The patients and the controls were typed on the same panel of antisera by the same technicians.
HLA typing for the A, B, C and DR antigens was performed by the standard microlymphocytotoxicity test using antisera supplied for the Third Asia-Oceania Histocompatibility Workshop Conference, 19868). For the determination of HLA-DR antigens, B lymphocyte enrichment was performed by the nylon wool column technique9). The following specificities were included in this study: HLA-A1, 2, 3, 11, 24, 26, 29, 30, 31, w33, w36; HLA-B7, 8, 13, 14, 27, 35, 37, 38, 39, 44, w46, w48, w50, 51, w52, w53, w54, w55, w57, w58, w59, w60, w61, w67; HLA-Cwl, w2, w3, w4, w5, w6, w7, w8; HLA-DR1, 2, 3, 4, 5, w6, 7, w8, w9, 10.
The relative risk (RR) was determined for each antigen by Woolf’s or by Haldane’s formula. The significance of deviation was calculated by Chisquare analysis or, where appropriate, by Fisher’s exact test from 2 × 2 contingency tables. “Corrected” P-values (cp) were derived by multiplying by the number of HLA specificities being investigated at each HLA locus.
RESULTS
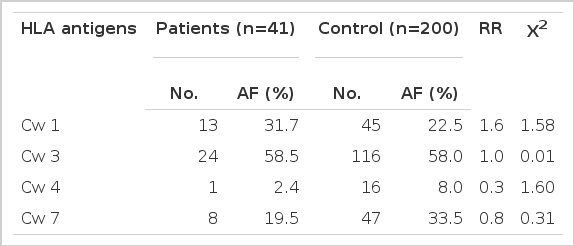
The HLA-A and -C alleles did not demonstrate significant differences in the frequencies between the patient and control populations (Table 1 and 3).
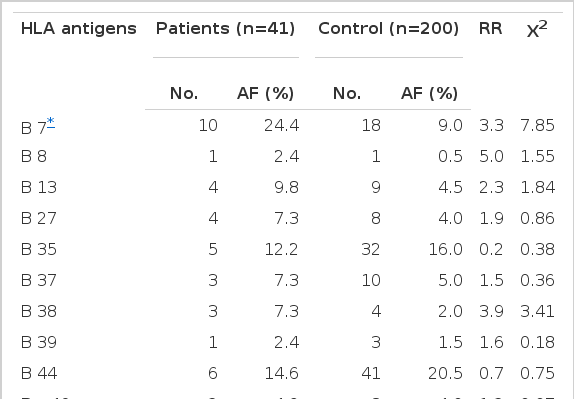
The frequencies of HLA-B7 and Bw60 were increased (24.4% vs 9.0%, RR 3.3, p < 0.01 for B7; 29.3% vs 13.0%, RR 2.8, p < 0.01 for Bw60) and the frequencies of Bw61 and Bw62 were decreased in the IDDM group (2.4% vs 17.5%, RR 0.1, p < 0.025 for Bw61; 2.4% vs 16.5%, RR 0.1, p < 0.025 for Bw62) as compared with the controls, but none was significant when P-value was corrected for the number of antigens tested (Table 2).
The frequencies of HLA-DR specificities in the IDDM and control populations are listed in Table 4. The frequencies of HLA-DR3 and DR4 in the patients with IDDM were significantly higher than those in the controls (19.5% vs 4.3%, RR 5.4, cp < 0.005 for DR3; 61.0% vs 36.4%, RR 2.7, cp < 0.05 for DR4). On the contrary, DR2 was decreased in the patients as compared with the controls (9.8% vs 32.1%, RR 0.3, cp < 0.05). There was a slight decrease of DRw6 frequency in the patients, 2.4% compared with 16.8% of 280 normal controls, but this decrease was not significant (p < 0.025).
DISCUSSION
In the present study, HLA-DR3 and DR4 had significant positive associations and DR2 had a significant negative association with IDDM. Also there was no difference in the incidence of DRw9 between the patients (12.2%) and the controls (21.1%). Amazingly, these data are similar to the results found in several diabetic populations of the European and North American Caucasians1). However, our results are different from the results found in the Japanese, Chinese and North Indian populations4, 10–13). These findings emphasize the difference in HLA association with IDDM among different ethnic groups and this ethnic variability in HLA-IDDM associations may suggest that the association is not primarily with HLA-DR locus but rather, primarily, with other genes (primary disease-promoting genes of IDDM) that are linked to the HLA-DR locus.
Interestingly, we also found an decreased frequency of DRw6 in Korean patients with IDDM, although it was not statistically significant. This result supports that DRw6 exert a possible protective influence on the development of IDDM that have been suggested in a study from Australian Caucasoid population by Kohonen-Corish et al14). In the past, the serotyping for accurate assignment of the DRw6 specificities has been difficult due to the lack of monospecific typing sera. Indeed, in the definitive study of HLA and IDDM in the Eighth International Histocompatibility Workshop, tabulations of DRw6 were omitted due to technical difficulties15). The possible protective role of DRw6 in IDDM may have previously overlooked. DR2 and DRw6 show similarities in their DQα and DQβ restriction fragment length polymorphisms, with different combinations of these occuring in different DR2 and DRw6 positive cells16). Further studies on the protective role of DRw6 in IDDM using serotyping and genotyping will be needed, we think.
In this study, we found a trend of increase in B7 and Bw60 and decrease in Bw61 and Bw62 frequencies in the patients with IDDM, although none reached statistically significant level when corrected P-value was calculated. This result was a contrast to HLA-B association with IDDM that had been found in other ethnic groups1). These differences suggest that the assoication of IDDM with HLA-DR locus is stronger than that with HLA-B locus. The ethnic difference of association with HLA-B locus can be explained by the different patterns of linkage disequilibria with the HLA-DR locus in different populations, if IDDM is the same in different populations.
Notes
This work was supported in part by speial research grants from Seoul National University Hospital (1987).