Subepidermal Capillary Basement Membrane Thickness of the Skin Obtained by Punch Biopsy in Patients with Non Insulin Dependent Diabetes Mellitus
Article information
Abstract
Thickening and proliferation of the capillary basement membrane is a generalized phenomenon in diabetes mellitus and has been described in many organs including the heart, kidney, pancreas, retina etc.
While such changes are specific, it is difficult to obtain specimens from those organs.
Tissue samples were obtained from the medial surface of the thigh of 33 diabetics and 4 healthy controls by means of punch biopsy.
Measurements carried out by normogram obtained from electron microscopic pictures.
HbA1c values were also determined at time of muscle biopsy.
The HbA1c values are higher in diabetics than in the control group (p<0.01).
The subepidermal capillary basement membrane thickness of the diabetics: 30% of the 5’th decade population, 53.9% of the 6’th decade population and 83.3% of the 7’th decade population was greater than 3,000 A°. Whereas that of the controls was less than 3,000 A°.
The subepidermal capillary basement membrane thickness was not significantly increased with the duration of the disease.
In cases of greater subepidermal capillary besement membrane thickness, HbA1c showed a significant increase. (p<0.01).
INTRODUCTION
The relationship between microvascular complications of diabetes mellitus and blood glucose level is unknown although this issue has been the subject of longstanding theoretical controversy1–3). Generally, microvascular diseases in diabetes are almost inevitably present in the kidneys and eyes, and it is known that the thickening of the capillary basement membrane is a characteristic finding of such lesions10,12,16).
But Siperstein et al.5), in 1968, reported that this characteristic lesion is also present in the capillary basement membrane of skeletal muscle of a high percentage of diabetic patients.
Conseduently many researchers then investigated changes in skeletal muscle capillary basement membrane thickness.
Kilo6), Siess et al.7) argued that capillary basement membrane thickening was not due to diabetes mellitus Itself, but rather to age, but studies such as Vrako’s8), Pardo’s9) Siperstein’s10), Danowski’s11), Raskin’s12) and Williamson’s13) etc. confirmed that capillary basement membrane thickening in skeletal muscle is significantly related to diabetes mellitus.
These researchers state that capillary basement membrane thickening in the eye, kidney and smooth muscle are characteristic changes, and futhermore, that observations of these changes are only be possible with tissues from these organs.
Since it is difficult to obtain tissue from these organs, the authors devised a method to sample skin tissue from the medial surface of the thigh by punch biopsy and then measured the subepidermal capillary basement membrane thickness with an electron microscope with reference to the method of Siperstein4) and Williamson16). Using this data the relationships between age, HbA1c level and duration of diabetes mellitus were determined.
SUBJECTS AND METHODS
1. Subjects
The subjects included 33 male and female patients, 30 years and older, who were admitted to Chosun University Hospital between August 1984 and May 1986 with diabetes mellitus. All subjects had fasting blood sugar levels greater than 140 mg/dl and post prandial 2 hour glucose levels greater than 200 mg/dl at the time of admission.
All subjects were classified according to the National Diabetes Data group (1979) protocol.
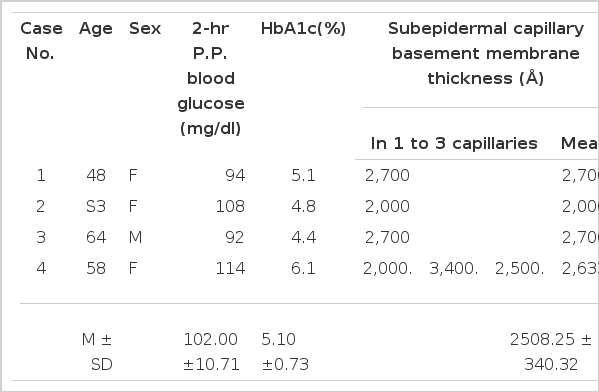
The control group consisted of 4 healthy subjects aged 40 years or older with fasting blood sugar and postprandial 2 hour blood glucose levels of less than 120 mg/dl and 115 mg/dl respectively.
2. Methods
Samples were taken from the medial surface of the thigh of each subject by means of a punch biopsy. The specimens measured 0.3 cm to 0.5 cm in length and 0.5 cm to 0.8 cm in depth, and were fixed in 25% glutaraldehyde solution and then in osmium tetraoxide for 2 hours, washed with caccodylate, dehydrated in graded ethanol, and embedded in an epon mixture by the Luft method.
Ultrathin sections were stained with uranyl-acetate and lead-citrate and examined under a JEM-100B electron microscope.
The presence of subepidermal capillaries in the sections was noted, the magnification was increased to 11,000, and they were photographed.
Siperstein14) obtained a mean value of 15 capillaries from each subject, while, we used an electronmicrograph to measure the basement membrane width of 1 to 3 capillaries per subject and calculated the real capillary basement membrane thickening with normograms17).
Blood glucose and hemolysated hemoglobin measurements were taken in each group. Fasting blood glucose and 2 hour post-prandial glucose levels were determined by the glucose oxidase method on an Abott autoanalyzer. The HbA1c test was performed on an ion exchange chromatographic column manufactured by Bio-Rad Co.
RESULTS
1. Age and Sex
The ages of the 33 diabetic subjects ranged from 30 to 75 years with the majority (69.7%) in the fifth and sixth decades, whereas the ages of the control group ranged from 48 to 61 years.
2. Age and Duration of Disease
The duration of disease included 19 cases (57.6%) of 1–3 years, 11 cases of 4–9 years and 3 cases of longer than 10 years, and the longest duration was 20 years in a 52 years old male (Table 3).
3. Duration, Postprandial 2 Hour Glucose Level and HbA1c Level
The diabetic subjects were divided into group according to the duration of illness i.e., 1–3 years, 4–9 years and over 10 years and the 2 hour postprandial blood glucose levels of these groups were on the average 403.37 ± 82.15 mg/dl, 268. 36±68.49 mg/dl and 362.33±159.07 mg/dl respectively.
The level of HbA1c in these 3 groups averaged 7.33 ± 0.98%, 7.93±1.56% and 9.03±0.63% respectively and were significantly elevated compared with that in the normal control group (4.90±1.56%) (p<0.01).
When comparing the levels of HbA1c in these 3 groups, those in the over 10 years group were significantly greater than those in 4–9 year group (p<0.01) and those in the 4–9 year group were significantly greater than those in 1–3 year group (p<0.05, Table 4), showing that the level of HbA1c increased with the duration of diabetes.
4. Subepidermal Capillary Basement Membrane Thickness
1) The results of the subepidermal capillary basement membrane thickness measurements
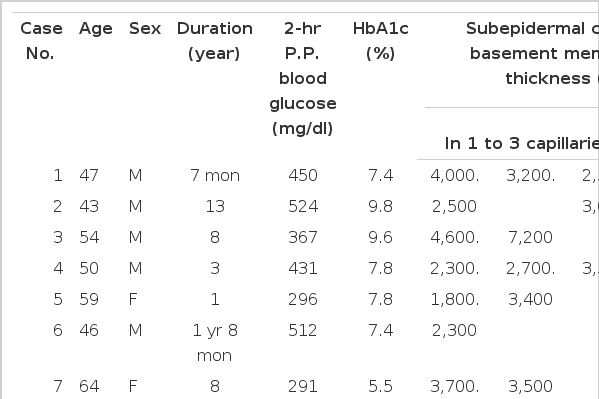
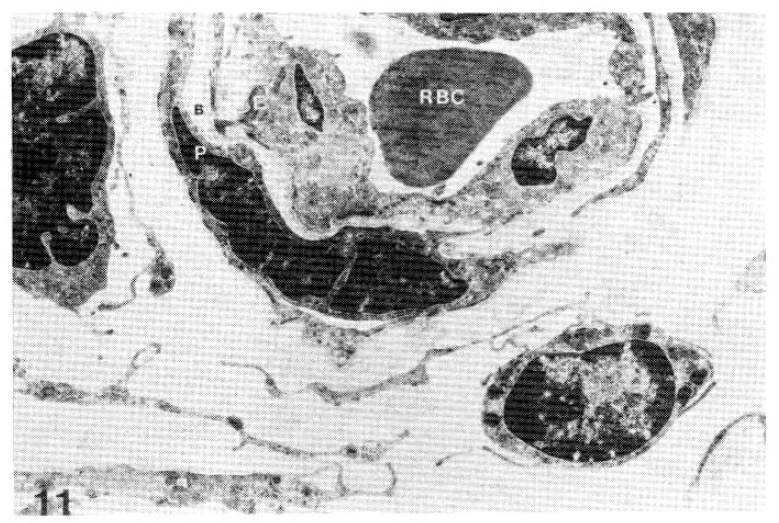
The results of the subepidermal capillary basement membrane thickness measurement in the 33 diabetic patients are presented in Table 1, shown in Figs, 4–10 and those of the control group are presented in Table 2, shown in Fig 11.
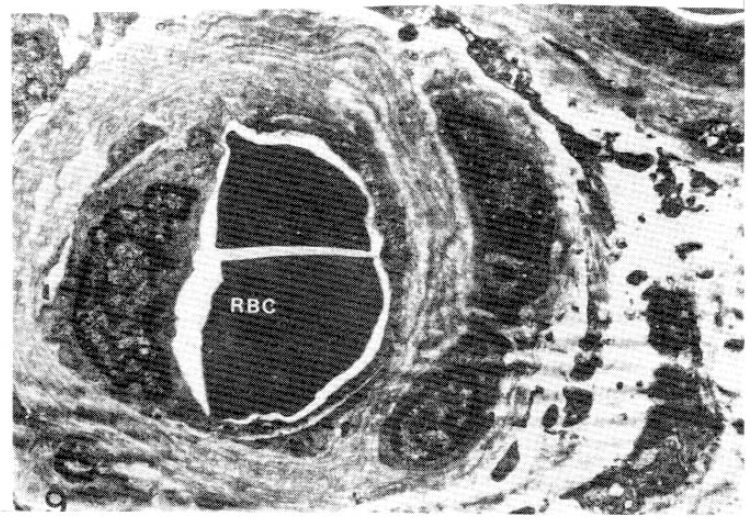
Case 5 (1 year duration of diabetes mellitus).
Two subepidermal capillary basement membrane thicknesses on electron micrograph measuring 2.0 mm and 3.5 mm In length, calculating 2,600 Å with normogram (×11,000).
Key for abbreviation.
RBC: Red blood cell, E: Endothelial cell, B: Basement membrane, P: Pericyte
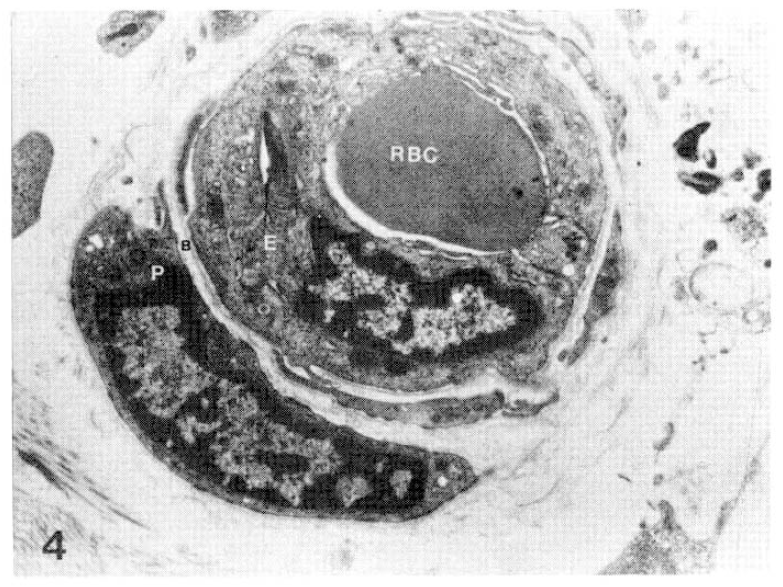
Case 24 (4 years duration of diabetes mellitus).
Two subepidermal capillary basement membrane thicknesses on electron micrograph measuring 5.5 mm and 9.0 mm in length, calculating mean 6.250 Å with nomogram (×11,000).
2) Comparison of subepidermal capillary basement membrane thickness according to age (Table 5)

Comparison of Subepidermal Capillary Basement Membrane Thickness (<3000 Å or > 3000 Å) and Duration in Diabetes
In 10 cases of 5’th decade diabetics, the subepidermal capillary basement membrane thickness was less than 3,000 A° in 7 cases (70%), and greater than 3,000 A° in 3 cases (30%), and the averages were 2,361.43±425.14 A°, and 3,533.33±378.59 A° respectively.
In 6 cases in the 4–9 year duration group, the average thickness was 2,305.00 ± 436.06 A°.
In 13 cases of 6’th decade diabetics, the subepidermal capillary basement membrane thickness was below 3,000 A° in 6 cases (46.1%), above 3,000 A° in 7 cases (53.9%) and the averages were 2205.50±648.15 A°, 6,568.86±4,403.99 A° respectively.
In 6 cases of 7’th decade diabetics, the subepidermal capillary basement membrane thickness was below 3,000 A° in 1 case (16.7%) above 3,000 A° in 5 cases (83.3%) and the averages were 2,400 A°, 4,043.60±1,872.26 A° respectively.
In the 4–9 year duration group the average thickness of 3 cases, in the 7’th decade with a subepidermal capillary basement membrane thickness of greater than 3,000 A°, was 3,233.33±317.54 A°.
In 29 cases of 5’th, 6’th and 7’th decade diabetics, the subepidermal capillary basement membrane thickness was below 3,000 A° in 14 cases (48.3%), above 3,000 A° in 15 cases (51.7%) and the averages were 2,297.36±501.91 A°, and 5,120.00±3,367.06 A° respectively (r: 0.7335, Fig 2).
3) Comparison of subepidermal capillary basement membrane thickness according to duration
In 10 cases of 5’th decade diabetics, the average subepidermal capillary basement membrane thickness in the 1–3 year duration, 4–9 year duration and over 10 year duration groups were 2, 418.57±498.75 A°, 3,200.00±707.11 A° and 3,800 A° respectively.
In 13 cases of 6’th decade diabetics, the average subepidermal capillary basement membrane thickness of the 1–3 year duration, 4–9 year duration, and over 10 year duration groups were 2,577.67±1,155.66 A°, 4,597.17±1,733.16 A° and 1,616 A° respectively.
In 6 cases of 7’th decade diabetics, the average subepidermal capillary basement membrane thickness of the 1–3 year duration, 4–9 year duration and over 10 year duration groups were 2,775.00±530.33 A°, 3,233.33±317.54 A° and 7,368 A° respectively (Table 6).
4) Comparison of subepidermal capillary basement membrane thickness and HbA1c level (Table 7)

Comparison of HbA1c and Subepidermal Capillary Basement Membrane Thickness (< 3000 Å or > 3000 Å) in Diabetes
In the 5’th decade diabetics, the average level of HbA1c was 7.30±0.77% in 7 cases (subepidermal capillary basement membrane thickness <3, 000 A°) and 9.30±1.32% in 3 cases (subepidermal capillary basement membrane thickness >3,000 A°).
In the 6’th decade diabetics, the average level of HbA1c was 7.35±1.14% in 6 cases (subepidermal capillary basement membrane thickness <3, 000 A°), 8.51±1.17% in 7 cases (subepidermal capillary basement membrane thickness >3,000 A°), and there was significant difference between them.
Also in 5 cases (6’th decade, subepidermal capillary basement membrane thickness <3,000 A°) of the 1–3 year duration group, it was 7.00 ± 1.02% (r: 0.7691, Fig 3).
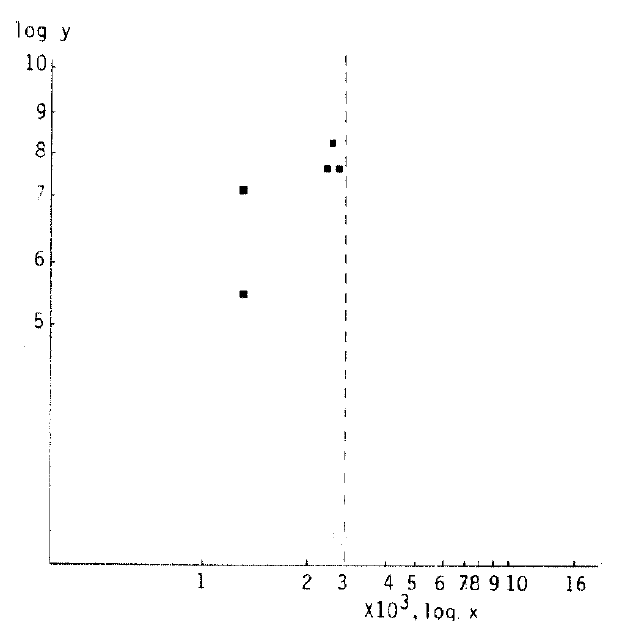
Correlation of HbA1c (log y) and subepidermal capillary basement membrane thickness (log x) in diabetes. In the 1 to 3 year-duration group and subepidermal capillary basement membrane thickness of 3000 Å or below in the 6’th decade population, the equation describing the regression line was log y = −0.4501 ± 0.3954 log x (r = 0.7691).
In the 7’th decade diabetics, the average level of HbA1c was 8.40% in 1 case (subepidermal capillary basement membrane thickness <3,000 A°), and 7.02±1.16% in 5 cases (subepidermal capillary basement membrane thickness >3,000 A°).
In 29 cases of 5’th, 6’th and 7’th decade diabetics, the average level of HbA1c was 7.40±0.93% in 14 cases (subepidermal capillary basement membrane thickness <3,000 A°) 8.17±1.42% in 15 cases (subepidermal capillary basement membrane thickness >3,000 A°), and there was significant difference between them (p<0.01).
DISCUSSION
The relationship between the microvascular complications of diabetes mellitus and blood glucose control is unknown although this issue has been the subject of longstanding theoretical controversy1–3).
In most tissues affected by diabetic microvascular disease, especially the eyes, kidneys, pancreas, retina and periadrenal capsule, a characteristic finding is a thickening of the capillary basement membrane4,12,16).
Small vessel lesions in diabetics are almost inevitably present in the eye, kidney, pancreas, retina and it is known that the thickening of capillary basement membrane is a characteristic finding of such lesion4,12,16).
But Siperstein et al., in 1968, previously reported that this characteristic lesion is also present in the capillary basement membranes of skeletal muscle in a high percentage of diabetic patients5).
Thus, the capillary studies of many authors concentrated on the capillary basement membrane thickness in the skeletal muscle.
Kilo6), Siess7) contradicted that duration of the diabetes mellitus is correlated to capillary basement membrane thickness, but that there is a relationship between capillary basement membrane thickness and age. However, Vrako8), Pardo9), Siperstein10), Danowski11), Raskin12), and Williamson13), have confirmed that capillary basement membrane thickness is correlated to the duration of the illness in the skeletal muscle of the diabetics.
In 1961, Aegenaes17) described a capillary basement membrane thickness in the skin of diabetics and then Handelsman18) and McMillan19) confirmed that basement membrane in the subepidermal capillary was thickened. Bercovici20) observed that the lesion is not qualitatively distinctive, because he observed similar capillary wall thickenings in skin biopsy specimen from nondiabetics.
However, usually in diabetics, we suggest that capillary basement membrane thickening is due to some observable causes.
That is, as regards pathogenesis, it is found that microangiopathic complications are correlated with blood glucose level1–3) and that capillary basement membrane thickening is due to microangiopathic change4,12,13). This microangiopathic change is also found in the subepidermal capillary basement membrane17,19,20).
Though it was believed that the lesion is not qualitatively distinctive in both of diabetics and nondiabetics20), we found in this study that capillary basement membrane thickening is not the same in diabetics and nondiabetics.
If differences in the subepidermal capillary basement membrane thickening in diabetics and nondiabetics were significant, then it would be possible for the clinician to use the skin punch biopsy method for practical purposes, as it is very difficult to obtain biological material from the eye, skeletal muscle and kidney, for diagnosis, mangagement and prognosis.
The first histologic study of cutaneous capillaries in patients with diabetes mellitus was that of Goldenberg et al.21) in 1959. He studied biopsy specimens from the skin of amputated legs: 92 from diabetics and 60 from nondiabetics.
He found the most prominent feature of vascular abnormality in the arterioles to be endothelial proliferation surrounded by concentric rings of reduplicated or spilt fibrils which were PAS positive.
In 1961, Aegenaes et al.17), studied the cutaneous capillaries of 33 patients, including 24 diabetics, with light and electron microscopy.
They found that the capillary walls of patients whose diabetes was detected before the age of 40 were thickened with a deposit of PAS positive material and that this thickening was absent or slight in patients without diabetes or in whom diabetes was detected after age 40.
In 1964, Bercovici et al.20), examined the cutaneous capillaries of diabetics and non diabetics and calculated the ratio of capillary wall thickness (CWT) to the size of the lumen diameter (LD).
The results were 0.502 in non diabetics, 0.64 in subjects with diabetes mellitus detected before age 40, 0.59 in subjects with diabetes mellitus detected after age 40, and 1.47 in subjects without diabetes mellitus but with dermatoses.
The thickness of the PAS stained band was graded and the results were 1.37 in non diabetics, 1.67 in subjects with diabetes mellitus detected before age 40, 1.45 in subjects with diabetes mellitus.
Detected after age 40, and 1.31 in subjects without diabetes mellitus but with dermatoses.
So, they reported that capillary wall thickening were present not only in diabetics but also in non diabetics.
But Bercovici, according to aforementioned method, measured subepidermal capillaries and found that capillary basement membrane thickening of the skeletal muscle is significant in diabetic patients using direct measurements of many capillary basement membranes thickness with electron microscope like Siperstein’s14) and Williamson’s15).
We also used a method of measurement with the electron microscope.
Glycosylation is an important aspect of hyperglycemia.
In this reaction, both in vitro and in vivo, glucose attaches to a protein via the free aminogroup at the N-terminus or E-amino group of lysine residues, forming a stable ketoamine derivative.
The interaction of glucose with the protein is not enzymatic, but is a condensation reaction in which the degree of glycosylation is proportional to the ambient glucose concentration22).
Glycosylation may alter the physiochemical properties of certain proteins, for example, glycosylation of hemoglobin affects its oxygen affinity thus preventing diffusion of oxgen into tissue, nonenzymatic glycosylation of a α-crystallins in the lens causes their aggregation and precipitation, and glycosylation of the capillary basement membrane leads to alterations in its structural and electrochemical properties manifested as increased permeability of the filtration barrier and inflow of collagen substance etc. into the basement membrane leading to its thickening22–26,34)
Generally, in clinical parctice, to demonstrate the degree of the glycosylation reaction, the levels of HbA1c are measured.
Also in this study the levels of HbA1c were measured in both the diabetic and control groups.
The results were 7.33±0.98%, 7.93 ± 1.56% and 9.03±0.63% in the patients of groups of 1–3 years, 4–9 years and over 10 years duration respectively.
In all diabetics, the level of HbA1c was significantly higher than that of the normal control group (4.90±1.56%) (p<0.01. p<0.01) and were significantly increased with duration (p<0.05, p<0.01).
This increase in the level of HbA1c was consistent with the data of Sosenko et al.27), and their data showed a strong positive correlation of muscle capillary basement membrane thickness to HbA1c levels in postpubartal type I diabetic patients.
Whether or not the glycosylation reaction is a direct factor in capillary basement membrane thickening is a matter of further investigation.
Paulsen et al.28,29), and Trivell et al.30), have show that the level of HbA1c is elevated 2–3 fold in diabetic patients.
Koening et al.31,32), reported that the increase in the level of HbA1c occurs 3–4 weeks after the onset of hyperglycemia but does not seem to be related to the severity or the duration of hyperglycemia, and that the level of HbA1c is not related to the blood glucose concentration nor to the thickness of the skeletal muscle capillary basement membrane.
Jaffe et al.33) reported that cultured human endothelial cells synthesize and release basement membrane collagen and that synthesis is increased especially in arteriosclerosis etc.
From this point of view, it is debatable as to whether or not an increase in glycosylation is related to vascular basement membrane thickening. However, considering the significant differences among the 3 groups in this study, together with the increase in HbA1c levels, this is a matter for further investigation.
In the diabetic group the average thickness of the subepidermal capillary basement membrane was 3554.18±2689.47 Å and the maximum was 16, 166Å in 52 year old patient.
This data illustrates the large variations among them as compared with that in the control group of 2444.23 ± 386.26 Å.
In subjects with diabetes mellitus detected after the age 40 the maximum and the minimum thicknesses were 7374 Å in a 53 year old patient and 2549 Å in a 56 year old patient respectively in the results of Dunn et al.35)
In the diabetic group there were 3 of 10 cases in the 5’th decade in which the subepidermal capillary basement membrane thickness was greater than 3000 Å and 7 of 13 (53.9%) in the 6’th decade.
Thickening of the quadriceps muscle capillary basement membrane was also observed In diabetics, more than 90% of the results of Siperstein et al.4) and more than 80% of the results of Aronoff et al.36) indicated that the thickening of the subepidermal capillary basement membrane is less than that of another site.
The comparison of subepidermal capillary basement membrane thickness in the diabetic group is presented in Table 5.
In the 5’th decade, the averages of the cases below 3000 Å or above 3000 Å were 2361.43±425. 1 Å and 3533.33±378.59 Å respectively, and that in the group of 1–3 years duration was 2305.00±436.06 Å.
In the 6’th decade, the averages of the cases below 3000 Å or above 3000 Å were 2205.50±648. 15Å and 6568.86±4403.99Å respectively.
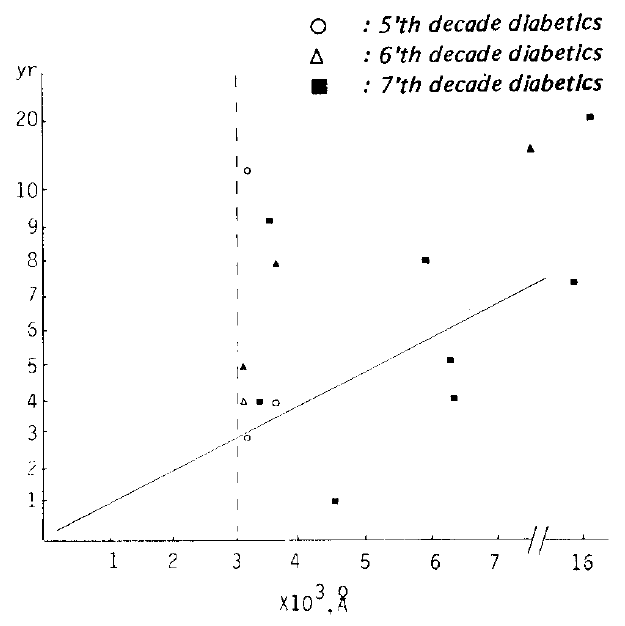
In the 7’th decade, the average of the cases below 3000 Å or above 3000 Å were 2400 Å and 4043.60±1872.26 A respectively, and in the same decade, the average of cases in which the thickness was above 3000Å and the duration 4 – 9 years, was 3233.33+ 317.54 Å (r: 0.9773. Fig. 1).

Correlation of duration (y) and subepidermal capillary basement membrane thickness (x) In diabetes. In the 4 to 9 year duration group and the subepidermal capillary basement membrane thickness of 3000 Å or above In the 7’th decade population, the equation describing the regression line was y = −14.9091 ± 6.3636 E−03x (r = 0.9707).
In a total of 29 cases of diabetes in the 5′th, 6′th and 7′th decades, the average of the cases, in which the subepidermal capillary basement membrane thickness was below 3000 Å or above 3000 Å, were 2297.36±501.91 Å and 5120.00±3367.06 Å respectively (r: 0.7335 Fig. 2).
In the study of Dunn et al.35) the thickness of the capillary basement membrane of skeletal muscle was 4434±1423Å in diabetics and 2951±1202Å in non diabetics.
The results of the research by Dunn et al.35), which is contrary to the findings of Siperstein et al., and Kilo et al., is related to changes in basement membrane thickness in retinopathy.
In particular these changes were calculated excluding the thickness of basement membrane due to increasing age.
Dunn opposes Kilo’s view which states that the basement membrane in retinopathy and that of skeletal muscle capillary basement membrane thickness are approximately the same, up to 90%35)
This study was designed to check the subepidermal capillary basement membrane thickness due to an increase in age, but in fact it includes a comparison with a similar aged control group. Our findings suggest that there may not be any significant difference due to an increase in age.
Changes in subepidermal capillary basement membrane thickness (A°) according to duration (1–3 years, 4 – 9 years, over 10 years) and age (decades) are presented in Table 6.
In patients of the 5′th and the 6′th decades, the subepidermal capillary basement membrane thickness of the 4 – 9 year group duration was increased as compared to that of the 1–3 year group duration, but not significantly so.
But the sudepidermal capillary basement membrane thickness of the group of over ten years duration was more increased than those of the 1 – 3 years and 4 – 9 years duration groups.
When the subepidermal capillary basement membrane thickness of all patients in the 5′th, 6′th and 7′th decades was compared, it increased with age but not significantly so.
Even though the site is different, the results of our study are similar to those of Dunn35): capillary basement membrane thickening of diabetes, has no significance to the duration.
But we suggest that the fact that the subepidermal capillary basement membrane thickening in the group over 10 years is comparatively different from the other groups should be a matter of further investigation.
Dunn et al.35) also insisted that age comparison is more significant than duration comparison in membrane thickening, but as we previously mentioned, our study does not show any significant increase.
We assume this fact also needs further investigation.
Comparison of HbA1c and subepidermal capillary basement membrane thickness in the diabetic group is presented in Table 7.
In the 5′th decade, the average level of HbA1c in patients whose average subepidermal capillary basement membrane thickness is below 3000 Å or above 3000 Å was 7.30±0.77% and 9.30±1.32% respectively.
In the 6′th decade, the average levels of HbA1c in which the average subepidermal capillary basement membrane thickness was below 3000 Å or above 3000 Å were 7.35 + 1.14% and 8.51 ±1.17% respectively, thus showing a significant difference between them (p<0.01).
In the group of 1–3 years duration in the 6′th decade, the average subepidermal capillary basement membrane thickness was below 3000 Å, and the average level of HbA1c was 7.00±1.0% (r: 0. 7691 Fig. 3).
In the 7′th decade, the average level of HbA1c in which the average subepidermal capillary basement membrane thickness was below 3000 Å or above 3000 Å were 8.40% and 7.02±1.16% respectively.
This data showed that the level of HbA1c decreased in cases in which the subepidermal capillary basement membrane thickness was above 3000 Å rather than in cases below 3000 Å. It may also be due to the small numbers of cases.
In 29 cases of diabetics, the average levels of HbA1c in which the average subepidermal capillary basement membrane thickness was below 3000 Å or above 3000 Å, were 7.40±0.93% and 8.17±1.42% respectively and there was significant difference between them (p<0.01).
Correlation of HbA1c and subepidermal capillary basement membrane thickness was calculated with logarithms. In the 1–3 year duration group and in which the capillary basement membrane thickness was below 3000Å in the 6′th decade population, correlation was significant (Fig. 3).
However, Koening et al.31,32) said that the above mentioned correlation was not good. In our view, we think that the overall correlation in the HbA1c and subepidermal capillary basement membrane thickness would be good, if our present study increased more subjects.
In conclusion the author, in presenting the above mentioned data and a more detailed investigation into the subepidermal capillary basement membrane thickness in patients with non insulin dependent diabetes mellitus, proposes that a practical direction for management and prognosis will be provided.
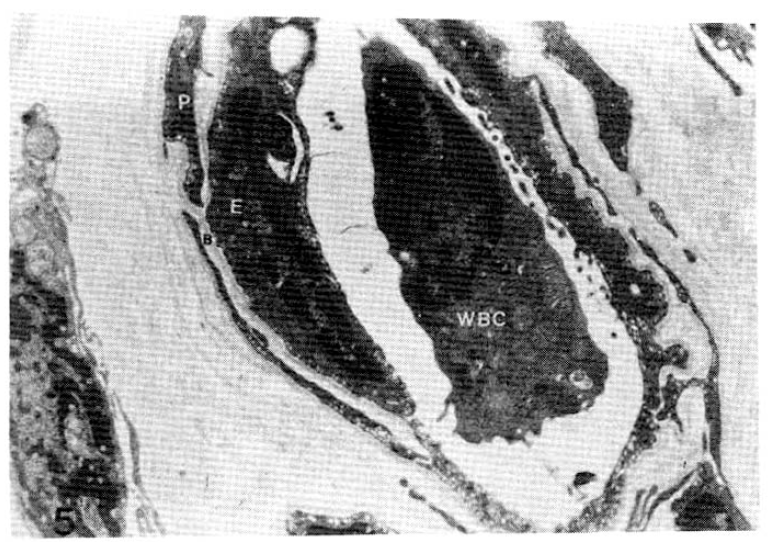
Case 6 (1.8 years duration of diabetes mellitus).
Average subepidermal capillary basement membrane thickness on electron micrograph measuring 2.5 mm In length, calculating 2,300 Å with nomogram (×11,000).
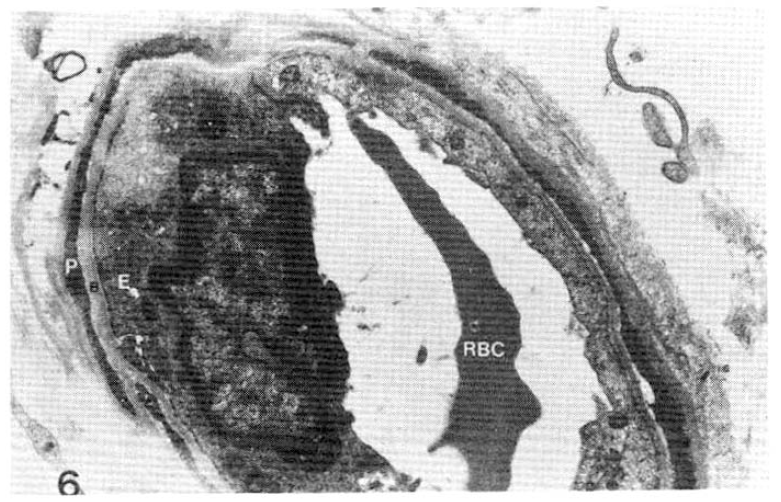
Case 15 (2 years duration of diabetes mellitus).
Average subepidermal capillary basement membrane thickness on electron micrograph measuring 2.5 mm in length, calculating 2,300 Å with normogram (×11,000).
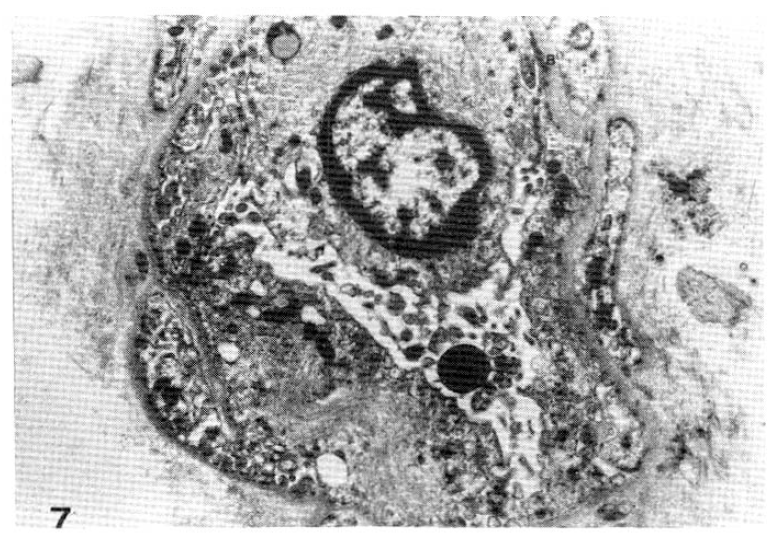
Case 30 (2 years duration of diabetes mellitus).
Two subepidermal capillary basement membrane thicknesses on electron micrograph measuring 1.3 mm and 1.8 mm In length calculating mean, 1,550 Å with normogram (×11,000).

Case 4 (3 years duration of diabetes mellitus).
Three subepidermal capillary basement membrane thicknesses on electron micrograph measuring 2.5 mm 3.0 mm and 4.0 mm In length, calculating mean 2,833 Å with normogram (×11,000).