Impaired Polymorphonuclear Leukocyte Function in Chronically Hemodialyzed Patients with Iron Overload
Article information
Abstract
Polymorphonuclear (PMN) leukoyte function tests and clinical data analyses were performed in 28 chronic renal failure (CRF) patients receiving regular hemodialysis. We divided them into two groups; 12 patients with normal serum ferritin were classified as group 1 and 16 patients with high serum ferritin as group 2. There was no difference in age, BUN, serum creatinine, complement (C3, C4), peripheral white blood cell count and the duration of dialysis between the two groups, but the serum iron level was higher in group 2 (129.1 ± 46.58 μg/dl) than in group 1 (74.3 ± 20.9 μg/dl) (p<0.001). The total iron binding capacity was lower in group 2 (p<0.05) and the number of transfusions was higher in group 2 (25 ± 16.1) than in group 1 (12 ± 8.7) (p<0.05). The nitroblue-tetrazolium (NBT) test showed no difference among groups 1, 2 and the healthy control group. In chemotaxis to fMLP (N-formylmethionylleucylphenylalanine), the mean number of migrated neutrophils to fMLP (10−6 M/L) per high power field was significantly decreased in group 2 (99.5 ± 37.6) compared with the healthy control group (140.1 ± 13.4) (p<0.005), but not in group 1 (155.8 ± 79.4). In the phagocytosis test using Staphylococcus aureus, the phagocytic index ratio compared to the healthy control group was significantly decreased in group 2 (0.59 ± 0.14), but not in group 1 (0.97 ± 0.18). These results suggest that iron overload due to multiple transfusions in patients receiving regular long term hemodialysis may play a part in causing susceptibility to infection by impairing PMN leukocyte functions, especially chemotaxis and phagocytosis.
INTRODUCTION
There are conflicting data regarding PMN leukocyte function in CRF patients receiving regular hemodialysis. Impaired chemotaxis is a well documented functional defect in uremic leukocytes with resulting impairment of the acute inflammatory response and decreased delayed hypersensitivity.1–4) Among the factors that can alter leukocyte function, iron overload may play a critical role.5–15) We previously discussed increased serum ferritin as a tumor marker in various malignant tumors.16) Furthermore, many reports have revealed that excessive serum ferritin may alter the immune system5,11,17–19) such as suppression of T-lymphocyte response to mitogen10), and may play a part in the immunosuppression of cancer patients. We performed various leukocyte function tests to determine the effects of serum ferritin in uremic patients on maintenance hemodialysis and in a healthy control group.
MATERIALS AND METHODS
Twenty eight patients receiving long term regular hemodialysis at the National Medical Center were divided into two groups according to their serum ferritin values. Twelve patients with normal serum ferritin values (34–245 ng/ml) were classified as group 1, 16 patients with serum ferritin values above 435 ng/ml as group 2. The healthy control group was composed of 10 medical personnel.
Samples for the leukocyte function tests were obtained by a large bore needle with preservative free heparin before starting heparinization for dialysis. Serum ferritin was measured by radioimmunoassay using the Ferritin 125I Radioimmunoassay kit of CIBA-CORNING Diagnostic Corp (USA).
The NBT test was performed by modifying the method of Park et al.20–22) 0.1 % NBT in saline solution was mixed with the same volume of phosphate buffer and centrifuged at 1000 rpm for 10 minutes. The supernatant was mixed with the heparinized blood and incubated at 37°C for 15 to 30 minutes and left at room temperature for 10 minutes. The supernatant plasma was removed and the buffy coat was collected with a capillary, smeared and dried on a cover glass. Wright’s stain was done and the leukocytes which reduced the NBT dye were counted under the microscope.
The chemotaxis test was performed using the Neuroprobe 48 well microchamber method (Neuroprobe, Inc).23) A microchamber was formed by the top and bottom plates and polycarbonate filter paper with micropores 3 μm in size (25 × 80) was used.
PMN leukocytes were collected by the usual method described anywhere.23) The separated PMN leukocytes were resuspended in HBSS (Hank’s balanced salt solution) at a concentration of 2 × 106 cell/ml. The synthetic chemo attractant, fMLP, which was stored at −70°C, was diluted with HBSS containing 16 mMol of HEPES (N-2-Hydroxyethylpiperazine N’-2-ethanesulfonic acid). Each well was filled with 25μl of the above mixture and covered by a PVP free membrane with micropore. This was then covered by a silicone gasket. The top plate well was filled with 40μl of PML leukocyte suspension (2 × 106 cell/ml) and the chamber was fixed and incubated in a 5% CO2 incubator at 37°C for one hour.
After incubation, the PMN leukocytes attached to bottom-well side of the filter paper were fixed with absolute methanol and stained by Gill’s hematoxylin method. The migrated leukocytes were counted in 5 high power fields (× 1000) and an average was obtained.24)
The dose response of the PML leukocytes migration according to the concentration of fMLP showed that HBSS with 10−6 M/l of fMLP at pH 7.2 induced the most migration. The chemotaxis study was done under the same conditions (Fig. 1.).
Phagocytic function was studied using Staphylococcus aureus ATCC 29273 in a concentration of 5 × 106 organisms per mililiter after over-night culture in broth. Serum of blood type AB was mixed with leukocytes for a final concentration of 5 × 106 cells per mililiter and the mixture was incubated with the bacteria at 37°C under continuous rotation (4 rpm) for one hour. Ice cold gelatin HBSS was added to stop phagocytosis and to sediment the leukocytes. The sediment was submitted to differential centrifugation for 8 minutes at 150g, and centrifuged again. Giemsa stain was done for morphological analysis.
The phagocytic index was determined by multiplying the number of leukocytes that phagocitized bacteria in 100 leukocytes by the average number of bacteria phagocitized in one leukocyte.
The phagocytic index of the healthy controls varied greatly probably according to subtle changes in experimental conditions. So, we calculated the ratio of the patient’s phagocytic index to that of the healthy controls on the same experimental day, and the value was designated as phagocytic index ratio. The results of 10 patients and 5 healthy controls for five consecutive days are listed in Table 4.
RESULTS
Age, BUN, creatinine and peripheral blood WBC counts were not different between the two groups. Serum iron was significantly higher in group 2 than in group 1 (p<0.001). Total iron binding capacity was lower in group 2 (p<0.05) and the number of transfusions was higher in group 2 (p<0.05) (Table 1.). Serum complement, C3, level was 28.3 ± 11.0 mg/dl in group 1 and 28.4 ± 10.0mg/dl in group 2. Serum C4 level was 24.0 ± 10.7 mg/dl in group 1 and 25.7 ± 12.7 mg/dl in group 2. These levels were not significantly different between the two groups.
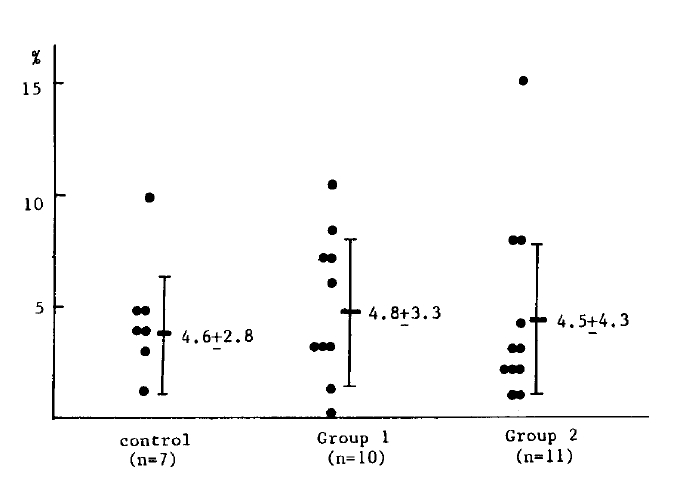
In the NBT test, the mean number of neutrophils that reduced NBT dye out of 100 neutrophils is 4.6 ± 2.8 in seven healthy controls, 4.8 ± 3.3 in ten patients in group 1 and 4.5 ± 4.3 in eleven patients from group 2. There was no significant difference among the healthy controls and the two patient groups (Table 2) (Fig. 2).
In chemotaxis, the mean number of migrated leukocytes was 140.1 ± 13.4 in 7 healthy controls, 155.8 ± 79.4 in six patients from group 1 and 99.5 ± 37.6 in fourteen patients of group 2. The number of migrated leukocytes in group 1 was not significantly different from the healthy controls, but that of group 2 was significantly smaller than that of the healthy controls (Table 3) and none of the patients in group 2 showed an increased number of migrated leukocytes in contrast to the variable counts in group 1. (Fig. 3).
In the phagocytosis test, phagocytic index ratios were lower in group 2 (0.59 ± 0.14) compared with group 1 (0.97 ± 0.18) (p<0.001) (Table 4∼5) (Fig. 4).
DISCUSSION
Alterations in the immune mechanism in uremic patients on maintenance hemodialysis have been reported by many authors, but the causes of immunologic defect have not yet been clearly defined. Moreover, there are many conflicting data about leukocyte function in patients with uremia.3,25)
It is known that NBT reduction capacity is not decreased in uremic patients but is increased during hemodialysis. Winchester et al. asserted that the predialytic increase of NBT reduction was related to systemic bacterial infection.5,21)
Chemotaxis was usually impaired in uremia. Granulocyte adherence,26) random motility cellular chemotaxis and serum chemotactic activity were decreased1,2,24,27,28,29) with resultant impairment of the acute inflammatory response and delayed hypersensitivity reaction.3,4) Casciani et al. reported increased serum chemotactic inhibitory activity in uremia.3,30)
Phagocytic function was also reported to be decreased in the uremic patient.1,5,25,31,32) But conflicting data showed that phagocytosis was not impaired in these patients.33,34)
In hemodialysis patients, repeated transfusion tends to result in iron overload,6) which increases and aggravates the frequency of infection.35,36) Excessive iron is utilized for bacterial growth, increases bacterial virulence and exerts a deleterious effect on phagocytosis and the metabolic activity of leukocytes.9) Phagocytic oxidative metabolism is also reduced with iron overload.17)
In vitro suppression of lymphocyte blastogenesis in response to mitogen stimulation and alteration of lymphocyte and phagocytic activity by the presence of ferritin on the surface of these cells were suggested.10)
Purified recombinant human heavy-subunit ferritin was shown to have a suppressive effect on granulocyte, macrophage and erythyroid progenitor cells in vitro and the monoclonol antibody to heavy-subunit ferritin can inactivate the suppressive effect of ferritin on hematopoietic stem cells37)
The exact mechanism involved in inhibiting leukocyte function is not clear, but excessive serum ferritin may form excessive toxic hydroxy radicals which cause peroxidation of leukocyte membrane lipids.5,13,14) In addition, phagocytic activity was normalized after chelation of toxic iron by desferrioxamine.5) Sweder et al. reported the normalization of phagocytosis by adding desferrioxamine to an iron excess medium in which phagocytosis was inhibited.15) In this report, we performed leukocyte function tests according to the level of serum ferritin and found that chemotaxis and the phagocytic index ratio to healthy controls were reduced in patients with high serum ferritin. It is suggested that excessive serum ferritin impairs leukocyte functions, especially chemotaxis and phagocytosis.
To clarify the relation of serum ferritin to leukocute function, further study will be needed. First, correction of leukocyte function is to be assessed after reducing the serum ferritin by desferrioxamine in vivo. Second, leukocyte function tests with normal PMN cells in ferritin added serum should be performed.
In addition, if serum ferritin exerts a deleterious effect as in our study, then increased ferritin as a tumor marker in various malignant diseases16) may be regarded as a cause of immune suppression in cancer patients also.