Clinical Review of 20 Cases of Addison’s Disease in Korea:
Including Previously Reported Cases and 6 New Cases at the National medical Center
Article information
Abstract
Addison’s disease is a rare disorder resulting from a chronic deficiency of adrenol cortical hormone. The clinical manifestations and data of 20 patients with Addison’s disease were reviewed. They include 14 previously reported cases, total cases in korea upto 1987, and 6 new cases at National Medical Center.
13 patients were male and 7 female. The mean age was 39 years and ranged from 16 to 61 years.
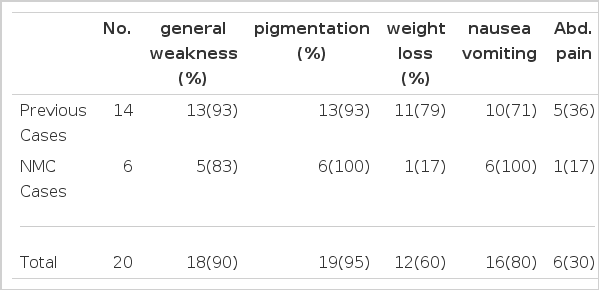
Common symptoms included mucocutaneous pigmentation (95 %), general weakness (90%), nasuea and/or vomiting (80%), and weight loss (60%).
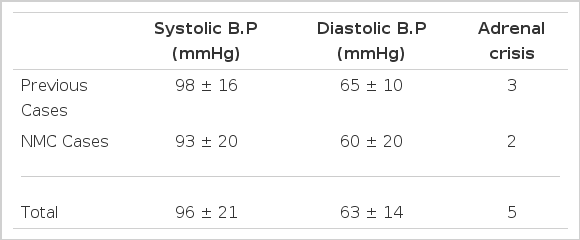
Initial mean blood pressure was 96±21/63±14 mmHg and adrenal crises were found in 5 cases (25%).
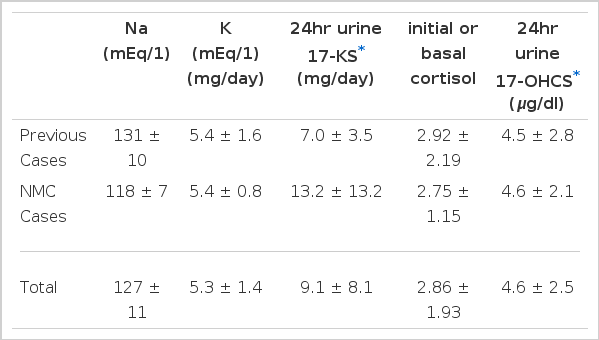
Laboratory data indicated hyponatremia and hyperkalemia, resulting in a decreased Na/K ratio. Serum basal cortisol and ACTH levels were 2.86±1.93 ug/dl and 482.5/269.3 pg/ml, respectively.
Extra-adrenal tuberculosis was present in 14 cases (70%) as a very high incidence.
These 14 cases (70%) were presumed to be due to adrenal tuberculosis, although only two cases were confirmed as such by histopathology. The other cases were likely to be non-tuberculous.
Thus, tuberculosis may be-considered as a predominant cause of Addision’s disease in Korea. Other features were not unusual. But further detailed and extensive studies are necessary including more pathologically confirmed cases.
INTRODUCTION
Addison’s disease is a rare discorder, characterized by primary adrenocortical hypofunction. It is usually caused by granulomatous destruction or idopathic atrophy of the adrenal cortex. In the United States, the most frequent cause is autoimmune destruction of the adrenal and the second is tuberculosis. However, tuberculosis remains the primary cause of the disease in many countries with a higher incidence of tuberculosis. Since the characteristics of Addison’s disease in Korea have not been evaluated, the authors reviewed 14 previously probably all reported cases ones in Korea upto December 1987 and 6 new cases seen at National Medical Center (NMC).
SUBJECTS AND METHODS
Between 1968 and 1987, 14 patients with Addison’s disease were reported in the literature in Korea. In addition, 6 new cases at National Medical Center were added to this study. A review of these case from the literature and medical records the basis for our analysis. There were 13 males and 7 females, ranging in age from 16 to 61 years, with a mean age of 39 years (Table 1).
1. Clinical Manifestations (Table 2)
The common clinical manifestations are shown in Table 2. The most common manifestation was mucocutaneous pigmentation (95%), and only one case showed no pigmentation. This 60 year old female presented extreme general asthenia, occasional nausea, anorexia and progressive weight loss during the year prior to admission. She had smear-positive cavitary pulmonary tuberculosis. Blood pressure was 100/70 mmHg, and hyponatremia and hyperkalemia were noted. Basal Cortisol and 8 hour intravenous ACTH test were normal. But Thorn’s test was positive and clinical improvement was seen with glucorticoid and other therapies. Thus the diagnosis of Addison’s disease was not confirmative but suggestive. In this case, however, a technical error during testing was suspected.
Other frequent manifestations were general malaise (90%), nausea and/or vomiting (80%), and weight loss (60%) in decreasing order.
2. Initial Blood Pressure and Adrenal Crises (Table 3)
The mean initial blood pressure (BP) of the subjects were 96±21/65±10 mmHg and the blood pressure of one patient was initially unmeasurable shock. The clinical features of adrenal crisis were seen in 5 cases, two of which were from the NMC. One unusual case presented a rifampin-induced adrenal crisis during concurrent treatment with rifampin arid glucocorticoid hormone due to Addison’s disease with adrenal tuberculosis.
3. Laboratory Findings (Table 4)
The serum sodium level was reduced to 127 ± 11 mEg/L and the serum potassium level increased to 5.3 ± 1.6 mEg/L. Thus the Na/K ratio was elevated as expected. 24hr urinary 17-hydroxycorticosteriod and 17-ke tosteriod levels were 4.6 ± 2.5 mg/24hour and 7.0±3.5 mg/24hour, respectively. The mean concentration of initial or basal serum cortisol was 2.86±1.93 ug/dl in 13 cases.
The level of serum cortisol at 4:00 PM was 2.5±1.5 ug/dl and ACTH level was 482.5±269.3 pg/ml. Renin and aldosterone levels were not checked in most cases. ACTH stimulation tests were positive in most cases (90%) with various methods (intravenous infusion test-8hours, 24hours etc, intramuscular injection). The negative results were attributed to technical or clinical errors.
4. Etiology and Concurrent Tuberculosis
Fourteen cases (70%) were clinically presumed to be due to tuberculosis due to concurrent adrenal and/or extra-adrenal tuber culosis.
Two cases which were confirmed as adrenal tuberculosis by histopathology, exhibited adrenal caseation and granulomatous lesions.
Two other cases presented adrenal calcification on plain X-ray or abdominal computed tomography. The organs with extra-adrenal tuberculosis were lung (60%), lymph node (10%), spine (5%) psoas (5%) and kidney (5%).
Six cases showed no evidence of tuberculosis and these were considered non-tuberculous, such as idiopathic atrophy etc. Also there was no combined endocrinopathy, other autoimmune disorder or significant illness.
5. Clinical Course
All patients showed good response to corticosteriod and other conservative treatment with clinical improvement. None of the patients expired during hospitalization although the results of long-term follow-up were not obtained.
DISCUSSION
The major etiologies of Addison’s disease are adrenal tuberculosis and idiopathic adrenal atrophy. All other causes are rare. The main concern of Addison’s disease is the ratio of its etiology to incidence which is variable according to era and region. As the demonstration of adrenal histopathology is not easily available, the causes of Addison’s disease are often obscure without adrenal biopsy. In our 20 patients, adrenal tissue was obtained only in two cases, and both were due to adrenal tuberculosis. Concomittant tuberculosis was noted in 70% of the subjects and this indicates a very high incidence of tuberculosis. Other autoimmune disorders or endocrinopathies were absent. Adrenal tuberculosis was considered in 14 cases (60%) clinically including the 2 cases confriend by biopsy. Two other cases were accompained by adrenal calcification. Due to the above facts and the high prevalence of tuberculosis in Korea, the causative contribution of tuberculosis to Addison’s disease may be predominant in spite of the problem of a small number of cases and a few histopathologically proven cases. The prevalence of tuberculosis in Korea was 2.2% in 198514) Even though it was markedly decreased from 5.1 % in 1965, tuberculosis is still a major problematic disease. Tuberculosis patients number approximate 500,000 and smear and/or culture positive ones number 200,00014).
Extensive tuberculosis in nonadrenal sites in a patient with Addison’s disease has been considered an indication that tuberculosis also caused the adrenal disease. Many studies reported evidence of extra-adrenal tuberculosis in patients with adrenal tuberculosis. Guttman15) reported that tuberculosis was found only in the adrenals in 7 (3%) of 243 tuberculous Addisonian patients and that other patients had extra-adrenal tuberculosis. Vita16) also reported that 12 (76%) of 17 adrenal tuberculosis patients was relatively common. In the United States, granulomatous destruction of the adrenal was predominant in the past, but over 80 percent of the recent cases are due to autoimmune destruction.17) O’Donnell18) Addisonian cases among 9,000 autopies from 1929 to 1949; 7 cases had tuberculosis of the adrenal and only 1 case presented idiopthic adrenal atrophy. Guttman15) reported that 566 Addisonian cases consisted of tuberculosis (68%); destructive strophy (19%), Amyloidosis (2%); tumors, fatty lesions, venous thrombosis, arterial embolism and syphilis (10%). Thus tuberculosis was a predominant cause of Addison’s disease until the mid-1950’s.
Tuberculosis has decreased as the causative factor of Addison’s disease due to effective anti tuberculous therapy and the resultant decrease in the incidence of tuberculosis. Forsham19) stated that destructive atrophy was the cause in 55% of the cases and tuberculosis 40% in 1963. At Guy’s Hospital in London, idiopathic atrophy was the cause of 40 of 53 Addisonian cases from 1948 to 1969.20) Nerup21) also reported that idiopathic atrophy was 66% whereas tuberculosis was 17%, in 108 Addisonian cases from 1950 to 1972. Idiopathic atrophy of the adrenal glands is resumed to be an autoimmune disorder. Antibodies against adrenocortical cells are found in the plasma for 73 % of the women and 50% of the men with the idiopathic form of the disease, into in the plasma of patients whose disease is due to tuberculosis.20)
There is lymphocytic infiltration of the glands. Antibodies against other tissues including the ovary, testis, placenta, gastric parietal cells, thyroid cells, and thyroglobulin are frequently found. Multiple autoimmune disorders may be associated with the idiopathic type of Addison’s disease. Other rare causes, such as fungal infection can induce granulomatous destruction of the adrenal, but there are no reported cases in Korea.
Another approach to the cause of the disease reviews the indirect findings of the disease. When adrenal tissue is not available, helpful clues include the duration of the disease, the size of the adrenal glands on abdominal computed tomographic scan and the presence of adrenal calcification. Radiographic detection of calcification in the adrenal glands of subjects with Addison’s disease has historically been regarded as a sign that strongly suggests adrenal tuberculosis and excludes idiopathic adrenal atrophy. Vita16) reported that calcification on abdominal imaging examination or on histopathologic examination was found in nine of 17 patients (53%) with adrenal tuberculosis. But one of the 16 patients with idiopathic atrophy had radiologic or histologic evidence of adrenal calcification. Rarely, other processes have been described as causing adrenal calcification. These include histoplasmosis, blastomycosis, hemorrhage and metastatic melanoma. Detection of adrenal calcification is helpful because it rules out idiopathic adrenal atrophy. So the absence of adrenal calcification does not always exclude adrenal tuberculosis.
Englargement of the adrenal glands varied both with the cause of adrenal disease and with the duration of adrenal insufficiency. The patients with adrenal tuberculosis for relatively short periods had enlarged adrenals, whereas those with a longer duration of disease had small adrenal glands.
In addition to early tuberculosis, other rare causes of adrenal insufficiency are associated with adrenal enlargement. There include histoplasmosis, blastomycosis cocidiodomycosis, cytomegalovirous, amyloidosis, adrenal hemorrhage, sarcoidosis, Hodgkin’s disease and metastatic carcinoma.16)
Because computed tomographic scan detects adrenal enlargement with great sensitivity, it has been proposed as a diagnostic tool in an effort to determine the cause of adrenal insufficiency. Small glands generally indicate either idiopathic atrophy or long-standing tuberculosis. The finding of enlarged adrenal glands on computed tomographic scan suggests the presence of early tuberculosis or in rare instances, other potentially treatable disease.16) Unless the clinical evidence of tuberculosis or malignant disease is convincing, the discovery of enlarged adrenal glands in a patient with Addison’s disease warrants serious consideration of adrenal biopsy.
In summary, tuberculosis was presumed to be the most frequent cause of Addison’s disease and the frequency of its presence was very high in spite of the small number of adrenal biopsies.
Unfortunately, computerized tomographic scans were performed in only a few cases (4 cases), so it is difficult to discuss the evaluation of the adrenal size and calcification which was undetectable on plain abdominal X-ray. Although a large portion of our cases with Addison’s disease was considered to be tuberculosis, an extended workup including computed tomography or adrenal biopsy is recommended to define the cause of Addison’s disease in Korea. In fact, many assessments of Addison’s disease were performed on the basis of adrenal histopathology or autopsy.
Other features except the high incidence of concurrent tuberculosis were not unusual compared with many previous reports. The relatively small number of cases is probably due to the fact that there were few biopsies or autopsies and infrequent medical help or studies. So we think that more detailed and extended studies and increased physician concerns about the disease are necessary due to the treatable characteristics of the disease.