ESHAP Salvage Therapy for Refractory and Relapsed Non-Hodgkin's Lymphoma: A Single Center Experience
Article information
Abstract
Background
The ESHAP chemotherapy regimen, that is, the combination of the etoposide, methylprednisolone, high-dose cytarabine and cisplatin, has been shown to be active against relapsing or refractory non-Hodgkin's lymphoma (NHL) in previous therapeutic trials. We attempted to determine whether ESHAP therapy would be effective and well-tolerated in Korean patients.
Methods
Twenty two patients with refractory or relapsed NHLs (all aggressive types) were enrolled in this study. We retrospectively evaluated the treatment response, the survival rate and the time to progression.
Results
Six patients (27.3%) attained complete remission and eight patients (36.4%) attained partial remission. The overall response rate was 63.6%. The median survival duration was 15.5 months (95% confidence interval; 10.7 to 20.3 months), and the median duration of the time to progression was 8.3 months (95% confidence interval; 0.3 to 16.3 months). Myelosuppression was the major toxicity, but severe neutropenia or thrombocytopenia was rare, and renal toxicity was also infrequent.
Conclusions
ESHAP regimen is effective in Korean patients suffering with relapsed or refractory NHLs, but a more effective salvage modality is needed because of the short duration of remission and the insignificant impact on long-term survival.
INTRODUCTION
The treatment of non-Hodgkin's lymphoma (NHL) has been considerably improved for attaining complete remission and long-term survival in approximately 50% of patients1-3). However, the prognosis of the patients who are refractory to treatment or who relapse with NHLs is dismal at best4, 5). Therefore, they need to be treated with effective salvage chemotherapy.
Until now, several multi-drug regimens have been suggested by numerous clinical trials, but the long-term survival with using these regimens was disappointing6-9). As an alternative way of cure using high dose chemotherapy (HDC) with subsequent autologous stem cell transplantation (ASCT) became a new strategy for treating refractory or relapsed NHLs10). However, we can apply this approach only to the patients who are young, who have a good performance status and who had a better response to prior chemotherapy. With these limitations, there are few randomized controlled trials about the efficacy of HDC with ASCT. So, an effective salvage regimen is needed for the majority of lymphoma patients who are old, who have a poor performance status or who had a prior history of chemotherapy resistance. A follow-up study that performed retrospective comparison of DHAP (the combination of dexamethasone, cytarabine, and cisplatin) and ESHAP regimens for treating refractory and relapsing lymphoma was recently published for the first time8, 11). Other authors have reported the later effects of an ESHAP regimen12, 13).
ESHAP is a combination chemotherapy regimen consisting of etoposide, methyl-prednisolone, cytosine arabinoside and cisplatin. The effect of cisplatin and cytosine arabinoside against NHLs as single agents was brief and the response marginal, and there have been reports about in vitro synergism between cisplatin and cytarabine14). With the background of these evidences, the activity of these two agents was tested in combination with etoposide as the ESHAP regimen and the response rate was remarkable (approximately 65%). Although in 1993 it was shown that there were no objective responses in 28 patients with refractory NHLs who received ESHAP16), the results of other trials later supported the effects of the ESHAP regimen.
There have been few studies about the effect of ESHAP therapy in Korean patients with refractory or relapsing NHLs15). Therefore, we wanted to determine whether this regimen would be effective and tolerable in Korean patients.
MATERIALS AND METHODS
Eligibility
Between January 1998 and November 2004, 22 patients who were treated at Asan Medical Center were considered eligible for this study. All patients had NHL that was refractory or they had relapsed after prior conventional chemotherapy. All patients had records of measurable biopsy-proven NHLs, and they had normal serum creatinine and transaminase levels. There were no known organ failures in these patients. The NHL was classified histologically according to the WHO classification: it was staged by the Ann Arbor classification and it was assessed via the International Prognostic Index (IPI). The patients had no active infections and no other malignancies. Before and after chemotherapy, involved field radiotherapy was allowed, if needed. All the patients gave us a written informed consent before starting the ESHAP regimen.
The combination of ESHAP
The ESHAP regimen consisted of etoposide (40 mg/m2, days 1-4), methyl prednisolone (500 mg, days 1-5), ara-C (2 g/m2, day 5), and cisplatin (25 mg/m2, days 1-4) (Table 1). In the other previous studies, cisplatin was administered as a continuous intravenous infusion due to the occurrence of cisplatin induced toxicity, but in the present study, we administered cisplatin as a 2 hours intraveonous infusion because of the patients' compliance. Each cycle was repeated every 3 or 4 weeks. Granulocyte colony-stimulating factor (G-CSF) was administered at a dose of 5 ug/kg/day by subcutaneous injection when the patient's absolute neutrophil count (ANC) was less than 500/mm3. Dose adjustments were made in accordance with the degree of myelosuppression. WHO grade 3 or more hematologic toxicity called for a 25% reduction of etoposide and ara-C.
Evaluation
The response to ESHAP salvage regimen was assessed after at least two courses of chemotherapy, and toxicity was assessed at every cycle. We used the standard WHO response and toxicity criteria. Treatment related death was determined when a patient died within 4 weeks of the initiation of the first cycle with no evidence of disease progression.
Statistical analysis
Overall survival (OS) was calculated from the first day of the first cycle of the ESHAP therapy to death or to the date of the last follow up. Time to progression (TTP) was defined as the duration from the time of treatment to the time of progression or to treatment-related death. The response duration was measured from the time of the first documented response to the time of relapse or progression in the patients who achieved complete remission or partial remission. Overall survival and the time to progression were calculated using the Kaplan-Meier method.
RESULTS
Patients
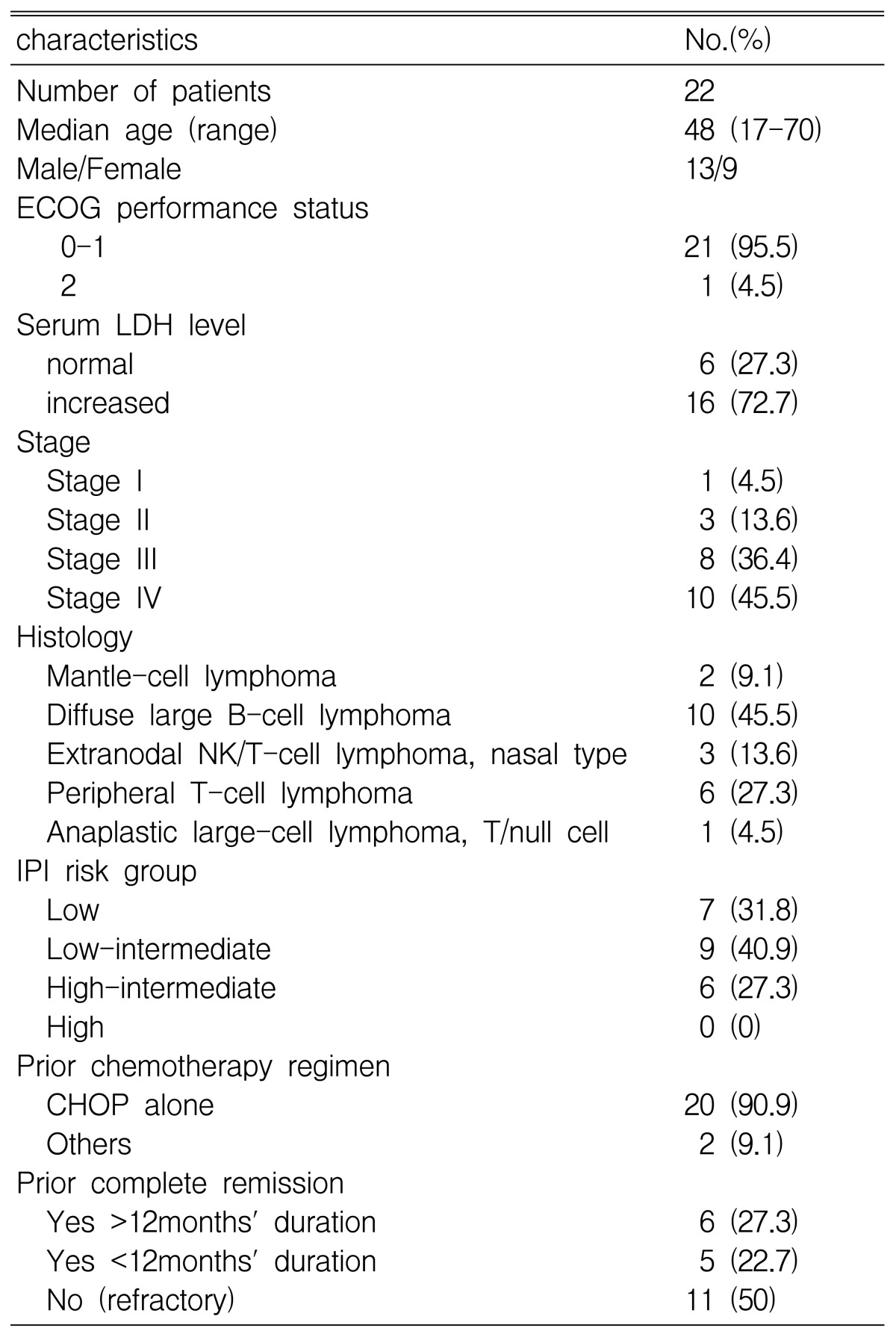
A total of 22 assessable patients with relapsed and refractory lymphoma received ESHAP. Among them, 13 were males and 9 were females (age range: 17 to 70 years). Of these patients, 21 (95.5%) had an ECOG performance status of 0 or 1. Before starting ESHAP therapy, the disease status was primary refractory disease in 11 (50%) patients and relapsed disease in 11 (50%) patients. The serum lactate dehydrogenase levels of 16 (72.7%) patients were elevated at the time of diagnosis. Of the 11 patients who had recurrent disease, 6 patients showed a prior duration of complete remission (CR) of more than 12 months. The histologic types of all patients were aggressive NHLs, which included mantle cell lymphoma (2 patients, 9.1%), diffuse large B cell lymphoma (10 patients, 45.5%), extranodal NK/T cell lymphoma, nasal type lymphoma (3 patients, 13.6%), peripheral T cell lymphoma (6 patients, 27.3%) and anaplastic large cell lymphoma (1 patients, 4.5%). The stage of the NHLs at the time of diagnosis was stage III in 8 patients (36.4%), stage IV in 10 patients (45.5%). The patient characteristics are listed in Table 2.
Response to ESHAP
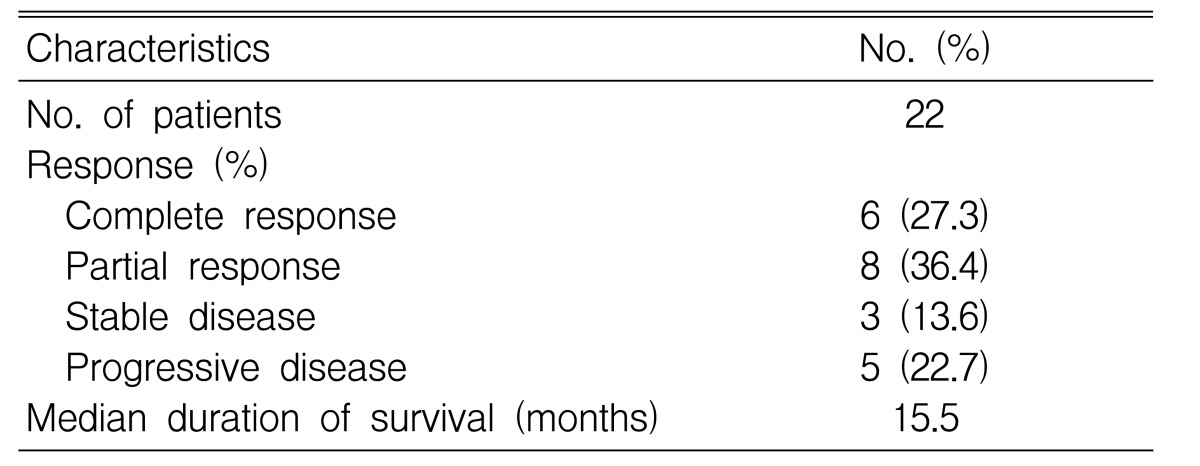
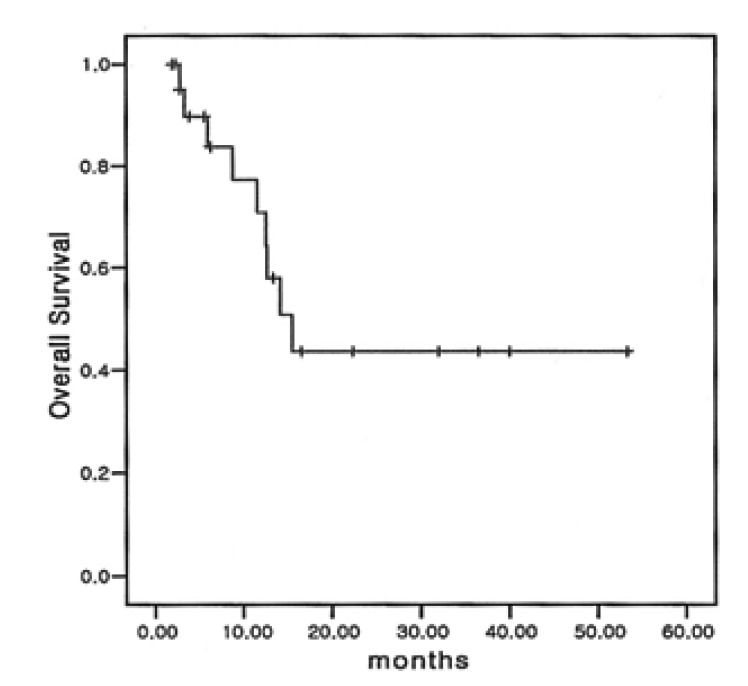
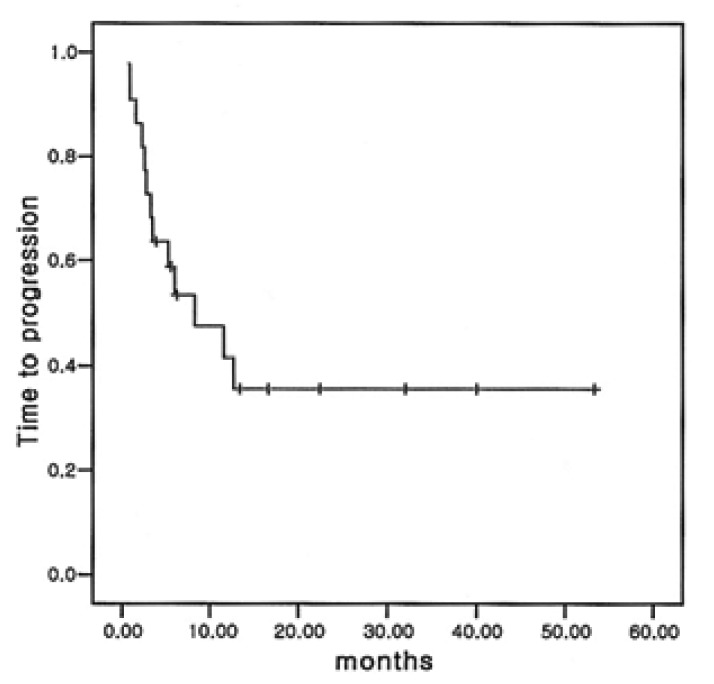
Among the 22 eligible patients, complete remission (CR) was achieved in 6 (27.3%) patients and partial remission (PR) obtained in 8 (36.4%) patients (Table 3). The overall response rate was 63.6%. Of the six patients with complete remission (CR), three had relapsed disease and another three had refractory NHLs to prior chemotherapy. A total of 70 cycles of ESHAP therapy were done for the 22 enrolled patients, with a range of one cycle to six cycles. The median duration of overall survival was 15.5 months (95% confidence interval; 10.7 to 20.3 months) (Figure 1) and the median duration of time to progression was 8.3 months. (95% confidence interval; 0.3 to 16.3 months) (Figure 2) The median response duration was 9.0 months (range 1.2 to 40 months).
Among the 22 patients, six (27.3%) patients underwent subsequent autologous stem cell transplantation (ASCT) after EHSAP therapy. In remaining 16 patients, we could not plan ASCT because 7 (31.9%) patients had disease with progression, 6 (27.3%) patients had treatment-related complication such as febrile neutropenia and pneumonia, 1 (4.5%) patient was lost to follow-up and 2 (9.1%) patients refused to receive ASCT.
Toxicity
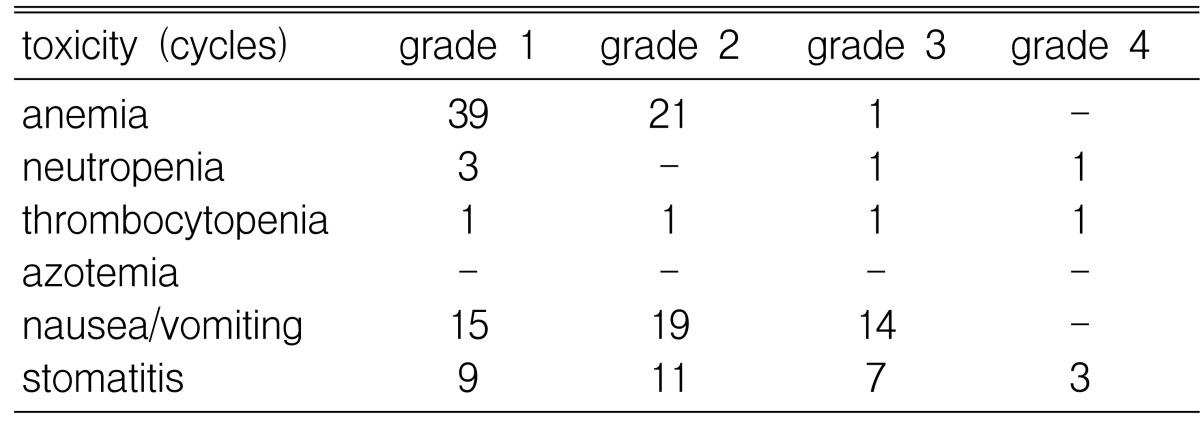
Hematologic toxicities were the main toxicities of ESHAP chemotherapy. WHO toxicity grade 3 neutropenia was observed during 1 (1.4%) cycle and grade 4 neutropenia was observed during 1 (1.4%) cycle. Grade 3 anemia was observed during 1 (1.4%) cycle and grade 3 thrombocytopenia was observed during 2 (2.9%) cycles. The major non-hematologic toxicities were nausea/vomiting (48 cycles, 69%), and mucositis (30 cycles, 43%). There were no treatment-related deaths or treatment-related azotemia (Table 4).
DISCUSSION
The widely accepted treatment modality for patients with advanced stage NHL is combination chemotherapy that is sometimes administered with radiotherapy. The previous conventional chemotherapy regimens have attained complete remission in about 50% of patients1-3). The best available regimen, the CHOP combination chemotherapy, has been widely used, but the prognosis of the partial responders to CHOP therapy is very poor with only 0~25% of patients becoming long-term survivors17). Therefore, there is a consensus concerning the need for effective and tolerated salvage chemotherapy for treating the refractory and relapsed patients. Since successful autologous bone marrow transplantation (ABMT) was first reported in 197818), several clinical trials have been designed to treat patients who have refractory or relapsed NHL with high dose chemotherapy (HDC) and ABMT. However, these strategies can be applied to only those patients who are young and who have a favorable general medical condition. The patients who are old or who are in a poor general medical condition need effective and tolerable salvage chemotherapy to obtain longer survival and a good quality of life.
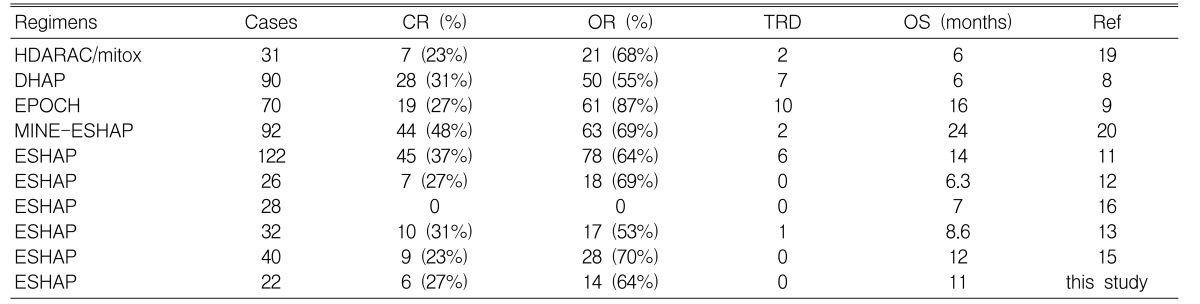
There are some previously published salvage regimens for refractory and relapsed NHLs such as IMVP-16 (ifosfamide, methotrexate and etoposide), MIME (methyl-prednisolone, ifosfamide, methotrexate and etoposide), EPOCH (etoposide, vincristine, doxorubicin, cyclophophamide and prednisone) and HDARAC/mitox (high-dose ara-C and mitoxantrone). In case of IMVP-16, this regimen attained 37% complete remission (CR), and the long term survival rate of thirty four total patients suffering with diffuse large B-cell lymphoma was 20%6). For the MIME regimen, among the 123 total eligible patients who had aggressive NHL, the complete remission rate and long term survival rate were 32% and 20%, respectively7). For the EPOCH therapy, there was a 27% complete remission rate in seventy patients9), and for the HDARAC/mitox regimen, there was a 23% complete remission rate in 31 patients19). In summary, the majority of the recent salvage regimens for refractory and relapsed NHL achieved about a 20% to 30% complete remission rate.
In 1988, Velasquez, et al. reported the results of the effect of DHAP (dexamethasone, high-dose ara-C, cisplatin) as salvage therapy for refractory and relapsed NHLs8). They also demonstrated the effects of a ESHAP regimen in 199411). Three years later, in 1997, they have followed up the two groups of patients; they stated that ESHAP was superior to DHAP for the overall survival and complete remission rates (For DHAP, the complete remission rate was 16%, and the median survival was 6 months. For ESHAP, the complete remission rate was 31%, and the median survival was 14 months). Although there were few long term survivors in both groups, ESHAP had a milder myelo-suppressive effect and a more beneficial clinical effect than did the DHAP regimen20).
However, in 1993, Johnson et al. reported the poor effect of ESHAP, which was in contrast to the study conducted by Velasquez el al.16) In the study by Johnson, of the 28 patients with refractory NHLs who received ESHAP, there were no objective responses and treatment-related toxicities were more common. In spite of the possibilities that different patients could have been selected for the different studies, the authors concluded that ESHAP showed limited efficacy, but marked toxicity in patients with refractory NHLs.
Rodriguez et al. have recently created a new regimen, MINE-ESHAP, which consists of mesna, ifosfamide, mitoxantrone and etoposide up to the maximum response, and this is followed by ESHAP consolidation. In their experience, this regimen had a higher response rate compared with ESHAP and DHAP21). Yet we should conduct a prospective randomized controlled trial in the near future to compare the differences between the efficacies of several salvage regimens.
More recently, new strategies have been introduced for improving the effect of relapsed and refractory NHLs; these include the ICE regimen, which consists of ifosfamide, carboplatin, etoposide, and the R-ICE regimen, which consists of ICE combined with rituximab22).
In our study, the complete remission rate and median survival was almost the same as that of the previous studies (Table 5). The reason for the longer median survival duration compared with the previous trials (15.5 months) seems to be the differences in the number of patients between the trials. For toxicities, there were also a small number of patients with severe neutropenia, thrombocytopenia, and anemia because the ECOG performance status of almost all the patients was 0 or 1. Prophylactic G-CSF (granulocyte colony stimulating factor) was another cause of the small number of toxic adverse events/effects. Moreover, since almost all the patients received chemotherapy in the outpatient clinic, the number of cycles of hematological toxicity probably doesn't exactly reflect the nadir of the blood count.
There are few reports about the effects of ESHAP for treating NHLs in Korean patients. In 2002, Choi et al. introduced the results of ESHAP therapy for refractory and relapsed NHLs in Korean patients21). The complete remission rate (23% vs 27% in our study), and the median survival (12 months vs 15.5 months in our study) showed no significant differences between the two Korean studies. This result suggests that the ESHAP regimen might be effective salvage chemotherapy for Korean patients, the same as it is for Western patients.
In conclusion, the ESHAP regimen is an effective and well-tolerated regimen for treating refractory and relapsed NHLs, but there are limitations that the duration of remission is brief and the impact on long term survival is insignificant. Further studies are still needed to determine the optimal regimens for treating refractory and relapsed non-Hodgkin's lymphoma patients.