A case of congenital bilateral coronary-to-right ventricle fistula coexisting with variant angina
Article information
Abstract
A coronary arteriovenous (AV) fistula consists of a communication between a coronary artery and a cardiac chamber, a great artery or the vena cava. It is the most common anomaly that can affect coronary perfusion. Yet bilateral involvement of a coronary fistula, constitutes an uncommon subgroup of coronary AV fistulas. We herein report on a case of bilateral coronary AV fistula that was coexistent with variant angina originating from the distal right ventricular branch of the right coronary artery and the distal septal branch of the left anterior descending artery, and the latter drained into the right ventricle.
INTRODUCTION
A coronary AV fistula consists of a communication between a coronary artery and a cardiac chamber, a great artery or the vena cava. In 1865, Krause1) described a congenital AV fistula, and this type of fistula makes up a part of the congenital anomalies of the coronary arteries, which occur in 1% to 2% of the population2). Such fistulas comprise 48.7% of all the congenital anomalies of the coronary arteries. Multiple fistulas occur in 10.7% to 16% of all coronary AV fistulas, whereas both coronary arteries are involved in about 5%2-4). This report describes the bilateral involvement of a coronary AV fistula, and this was coexistent with variant angina.
CASE REPORT
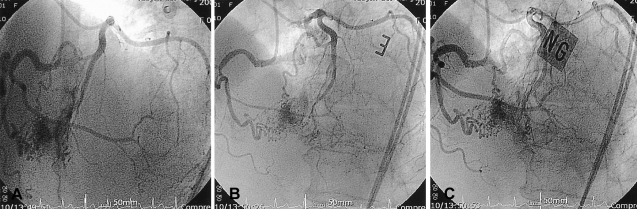
A 64-year-old woman presented with complains of progressive dyspnea on effort and also chest discomfort she'd had for 10 days. She has had no particular prior medical/surgical history. On the physical examination, the pulse rate was 70 beats/min and the blood pressure was 130/70 mmHg. Her heart sounds were normal and no murmur was heard. Abdominal palpation showed no abnormal findings and any bruit was not heard. The electrocardiogram displayed normal sinus rhythm; the chest X-ray showed no signs of pulmonary hyperemia or mass lesions, and the central shadow was normal (cardiothoracic ratio=0.49). The transthoracic echocardiography demonstrated a normal finding with good LV systolic function and the stress transthoracic echocardiography also showed no regional wall motion in the left ventricle. 24-hour holter monitoring showed no specific findings except for rare ventricular premature complexes (VPCs). There were no atheroma and stenotic lesions on the coronary angiogram, but it did reveal bilateral coronary AV fistula coexistent with vasospatic angina. One fistula originated from the distal septal branch of the left anterior descending artery and the other originated from a distal right ventricular branch of the right coronary artery and the latter drained into the right ventricle (Figure 1). The spasm study found that the middle to distal left anterior descending artery had diffuse 90% vasoconstriction with chest pain after a left intracoronary ergonovine injection. The vasoconstriction was relieved after a intracoronary nitroglycerin injection (Figure 2). She was discharged and then medicated with a calcium-channel blocker.

One fistula originated from the distal septal branch of the left anterior descending artery (A) and the other fistula originated from a distal right ventricular branch of the right coronary artery that drained to the right ventricle (B).
Baseline coronary angiogram before intracoronary ergonovine injection (A). The middle to distal left anterior descending artery showed diffuse stenosis with chest pain after left intracoronary 50 ㎍ ergonovine injection (B) and then this stenosis was relieved after intracoronary nitroglycerin 150 ㎍ injection (C).
DISCUSSION
For coronary AV fistula, the site of origin of the fistula is from the right coronary artery in 50% to 58% of patients, from the left anterior descending artery in 25% of patients, from the circumflex artery in 18.3% of patients, from the diagonal branch in 1.9% of patients and from the left main coronary artery (LMCA) or the circumflex-marginal branch in 0.7% of patients, respectively2, 3, 5, 6). In the above mentioned studies, the coronary AV fistula drainage site was the pulmonary artery in 29.8% to 43% of patients, the right ventricle in 14% to 40% of patients, the right atrium in 19% to 20.2% of patients, the left ventricle in 5.8% to 19% of patients and the left atrium in 5% of patients. Twenty percent of the patients with congenital coronary AV fistula have other congenital or acquired heart disease2-4, 7-9). For the adults with AV fistula, 60% complained of fatigue and dyspnea on effort. Angina pectoris and myocardial infarction that are due to coronary artery steal occur in 3% to 7% of AV fistula patients. Congestive heart failure develops in 19% of these patients, and endocarditis in the fistula occurs in 20%. The fistula may rupture spontaneously, causing hemopericardium and cardiac tamponade5). However, 40~50% of fistulas are totally asymptomatic7). Only one case has been reported with the coronary AV fistula coexistent with variant angina10).
The treatment of a coronary AV fistula depends on its presentation and the magnitude of the pulmonary-to-systemic flow. Yet it has been suggested that surgical ligation4), with or without the use of extracorporeal support, could be a form of treatment due to the natural history of the disease, which is not always benign, and also due to the low incidence of spontaneous fistula closure in patients with a large coronary AV fistula.
In conclusion, we report here on a case that showed the very rare occurrence of bilateral coronary AV fistula that was coexistent with variant angina.