Relationship Between Left Atrial Size and Stroke in Patients With Sinus Rhythm and Preserved Systolic Function
Article information
Abstract
Background/Aims
Increased left atrial (LA) size has been proposed as a predictor of poor cardiovascular outcome in the elderly. In the present study, we evaluated the relationship between LA size and stroke in subjects of all ages who presented with preserved left ventricular systolic function (LVSF) and sinus rhythm (SR), and investigated the relationships between LA size and other echocardiographic parameters of diastolic function.
Methods
A total of 472 subjects were enrolled in the study (161 men, 311 women) and divided into the stroke group (n=75) and control group (n=397). A conventional echocardiographic study was then performed. Subjects with valvular heart disease, atrial fibrillation, or coronary heart disease were excluded.
Results
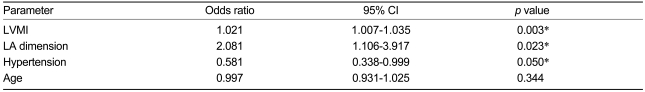
The mean subject age was 65.2±5.1 years in the stroke group and 65.6±5.9 years in the control group. Mitral inflow pattern and E & A velocity showed no significant relationship with stroke (p=NS, p=NS, respectively). Left ventricular mass index and LA dimension were significantly related to stroke (p=0.003, p=0.023, respectively), and hypertension showed a marginal relationship with stroke (p=0.050). Age was not related to stroke in the present study (p=NS).
Conclusions
The LA dimension is significantly related to the incidence of stroke. Therefore, strategies for prevention of stroke in patients with preserved LVSF and SR should be considered in cases of LA enlargement.
INTRODUCTION
Increased left atrial (LA) size is associated with poor cardiovascular outcomes such as the development of heart failure, atrial fibrillation (AF), and stroke in the elderly, even in cases in which sinus rhythm is maintained [1]. There is already strong evidence for an association between AF and ischemic stroke, and the majority of these strokes occurred in patients who presented with sinus rhythm [2]. Therefore, increased LA size has been suggested to be related to stroke in elderly people with sinus rhythm [3].
LA size is regarded as a reflection of the average effect of the left ventricular (LV) filling pressures against the LA over time and has been proposed as a surrogate marker of diastolic burden [4]. In addition, increased LV filling pressure resulting from a decrease in myocardial contractility or impairment of myocardial relaxation is considered the main pathophysiological factor involved in heart failure and contributes to enlargement of the LA [5]. To minimize the impact of LV filling pressure on LA size, the present study was performed in subjects presenting with preserved left ventricular systolic function (LVSF).
The present study was conducted to re-evaluate the relationship between LA size and stroke in subjects of all ages, not just the elderly, who presented with preserved LVSF and sinus rhythm, and to investigate the relationships between LA size and other echocardiographic parameters of diastolic function in these subjects.
METHODS
Subjects
A total of 472 eligible subjects (161 men and 311 women) were enrolled in this study between August 2006 and February 2007. The stroke group (n=75) consisted of cases of newly diagnosed first ischemic stroke attack confirmed by computer tomography or magnetic resonance imaging. The control group (n=397) consisted of healthy participants identified in an annual health evaluation program. Subjects with a LV ejection fraction <50% and valvular heart disease presenting with greater than mild insufficiency of the aortic or mitral valve, with any degree of stenosis of the valves, or with structural abnormalities were excluded from the present study. Patients with a history of coronary artery disease or Q-wave on surface ECG were also excluded.
Assessment of cardiovascular risk factors
Clinical data were obtained by a comprehensive review of each patient's medical record. Cardiovascular disease (CVD) risk factors adopted for the scoring method used in the Framingham Heart Study [6] were investigated for each patient based on age, gender, history of systemic hypertension, diabetes mellitus, hyperlipidemia, and smoking (Table 1). Systemic hypertension was defined by prior documentation of the clinical diagnosis or by evidence of elevated systolic blood pressure of 140 mmHg and/or diastolic blood pressure of 90 mmHg on two occasions, in the absence of acute medical illness. Diabetes mellitus was based on prior clinical documentation of the condition, regardless of whether the patient was being managed with dietary therapy, oral hypoglycemic agents, and/or insulin. Hyperlipidemia was defined by a fasting total cholesterol level >240 mg/dL and/or low-density lipoprotein cholesterol level >160 mg/dL on two occasions, or the use of cholesterol-lowering drugs. Current smoking was referred to as the active use of tobacco products at the time of enrollment in the study.
Echocardiographic examination
LVSF was assessed using both the semiquantitative visual estimation method and a modification of Simpson's method. Subjects with LVSF <50% on one or both methods were excluded from the study. The wall motion score index was also applied to exclude cases with regional wall motion abnormalities. The index was calculated by divid-ing the sum of the scores by the number of visualized segments.
Mitral inflow was assessed by pulsed-wave Doppler echocardiography from the apical four-chamber view, with the Doppler beam aligned parallel to the direction of flow and with a sample volume of 1-2 mm at the mitral tips. From the mitral inflow profile, E- and A-wave velocities, E-deceleration time (DT), and the E/A ratio were measured. Tissue Doppler imaging (TDI) from the apical four-chamber view was also performed, with a sample volume of 1-2 mm at the septal mitral annulus, to obtain the ratio between transmitral early peak velocity and early diastolic mitral annulus velocity, which was taken as the E/E' ratio.
Diastolic filling patterns were categorized as normal, impaired, or abnormal relaxation; pseudonormal; or restrictive, according to previous studies [7,8]. First, the mitral annular velocity was measured at the septal side using TDI, to determine the E'/A' ratio. The normal pattern of diastolic filling was defined as both E/A and E'/A' ratios greater than 1. Cases in which the E'/A' ratio and E' velocity were less than 1 and 7 cm/s, respectively, were defined as showing the abnormal relaxation pattern if the E/A ratio was less than 1, or the pseudonormal pattern if the E/A ratio was greater than 1 and less than 2.
LA size was assessed at end systole, just before the frame preceding mitral valve opening. The LA dimension was measured using its anteroposterior dimension obtained from the parasternal long axis view [9]. Based on the suggestion of Devereux et al. [10], the following equation was applied to determine the LV mass in g, where the mass can be determined from the LV short-axis dimension using a simple geometric cubic formula without measuring the major axis of the LV:
LVmass = 0.8×1.04[(LVID+PWT+IVST)3-LVID3]+0.6,
where LVID is the LV internal dimension, PWT is the posterior wall thickness, and IVST is the interventricular septal thickness. The LV mass index was obtained by dividing the LV mass by the body surface area.
Statistical analysis
To evaluate the association between stroke event and LA size, the data were classified into continuous variables (clinical and echocardiographic data) and categorical variables (CVD risk factors). Data for continuous variables are expressed as the means±SD. All measurements in the stroke and control groups were compared using an unpaired, two-tailed Student's t-test (SPSS version 12; SPSS Inc., Chicago, IL). Data were compared between subgroups within the stroke group and controls by oneway ANOVA. The values of the categorical variables were tested using the χ2 test. A value of p<0.05 was considered statistically significant.
To determine the relationships between LA size and the categorical and continuous variables that showed significance in the χ2 test between the stroke and control groups or in the univariate correlation analysis between significant parameters and LA dimension, logistic regression or linear regression analyses were performed, respectively. Univariate analysis of variance (general linear model) was applied to evaluate the degree of association between stroke and LA size, in addition to the above variables that indicated significant differences between the stroke and control groups or a significant relationship with the LA dimension on regression analysis. Significance was defined as p<0.05 for all analyses.
RESULTS
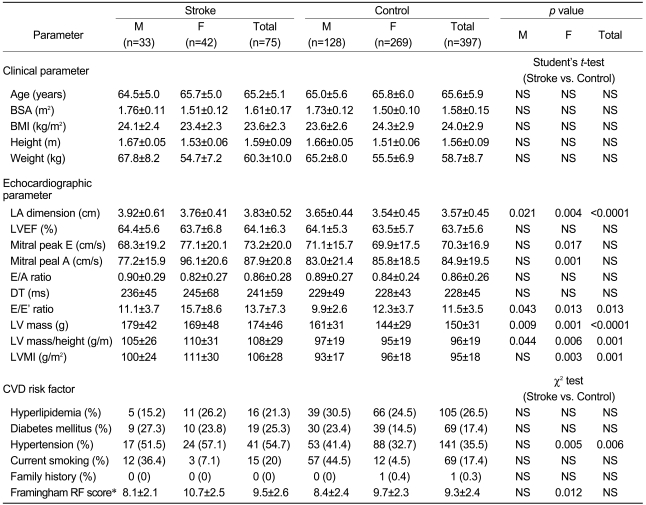
The mean age was 65.2±5.1 years in the stroke group and 65.6±5.9 years in the control group. There was no difference in mean age between the two groups, which were reclassified by gender. Other clinical parameters such as body surface area (BSA), body mass index (BMI), height, and weight were not significantly different between the stroke and control groups (Table 2, upper panel). Therefore, the possible bias for the LA dimension, which may occur due to differences in clinical parameters between the groups, was considered too minimal to affect the results of the present study.
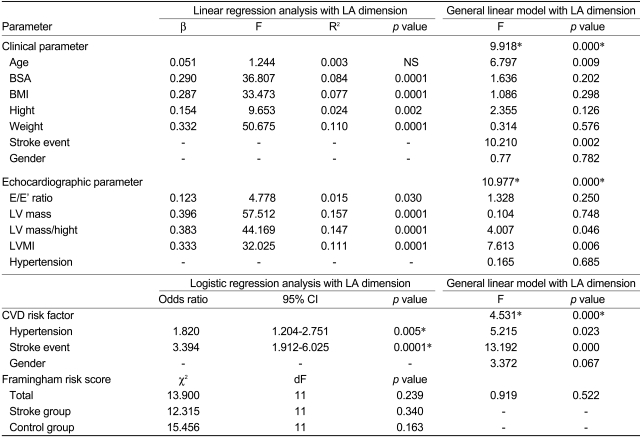
Regression analysis indicated that the clinical parameters, with the exception of age, showed weak positive correlations with LA dimension (BSA: r=0.290, p<0.0001; BMI: r=0.290, p<0.0001; height: r=0.287, p=0.002; weight: r=0.154, p<0.0001). Univariate analysis of variance revealed significant relationships of LA dimension with stroke and age, but no significant relationships of LA dimension with the other clinical parameters or gender (Table 3, upper panel).
Statistical analyses of clinical and echocardiographic parameters and CVD risk factors with LA dimension
Relationships between the LA dimension and cardiovascular risk factors
The incidences of systemic hypertension, diabetes mellitus, hyperlipidemia, and smoking were determined and compared between the stroke and control groups. There were no significant differences in the incidences of these CVD risk factors between the two groups, with the exception of hypertension, which showed a difference only in women (stroke vs. control: 57.1% vs. 32.7%; p=0.005; Table 2, lower panel). Therefore, univariate analysis of variance was performed to determine the relationship between stroke and LA dimension, using hypertension as a fixed factor. The LA dimension was strongly related to stroke, while a lesser relationship was seen with the history of hypertension (p<0.0001, p=0.023, respectively; Table 3, lower panel).
This study did not find a significant difference between the stroke and control groups with regard to the Framingham risk score (9.5±2.6 vs. 9.3±2.4, respectively; p=0.388). When the patients were regrouped according to gender, the Framingham risk scores for men did not differ between the stroke and control groups (8.1±2.1 vs. 8.4±2.4, respectively; p=0.46), whereas the scores for women did differ between the two groups (10.7±2.5 vs. 9.7±2.3, respectively; p=0.012). This finding was considered to be due to the relatively high incidence of hypertension among women in the stroke group in our study population (Table 2). Univariate analysis of variance revealed no association between the Framingham risk scores and LA dimension. The Framingham risk scores have been shown to be associated with various cardiovascular outcomes, but the scores did not directly reflect the anatomical changes of the LA in the present study.
Echocardiographic parameters in the stroke and control groups
The LA dimension of the stroke group (3.83±0.52 cm) was significantly greater than that of the control group (3.57±0.45 cm; p<0.0001). The LA dimension was greater in men than in women (3.70±0.49 vs. 3.57±0.46 cm, respectively; p=0.003) and was greater in men and women, respectively, of the stroke group compared with the corresponding controls (men: 3.92±0.61 vs. 3.65±0.44 cm, p=0.021; women: 3.76±0.41 vs. 3.54±0.45 cm, p=0.004). The LVEF of the stroke group was not significantly different from that of the controls, and the LVEF in men and women, respectively, did not differ between the stroke and control groups (Table 2, middle panel).
Peak E and A velocity of the mitral inflow were similar between the men of the stroke and control groups, but differed significantly between the women of the stroke versus control groups. The E/E' ratio, LV mass, LV mass/height, and LV mass index were significantly greater in the stroke group than in the control group. The E/A ratio and DT were not significantly different between the two groups. On regression analysis, these four echocardiographic parameters showed significant linear relationships with the LA dimension. Univariate analysis of variance using these four parameters and hypertension taken together indicated that the LV mass index was most closely associated with the LA dimension (p=0.006), followed by the LV mass/height (p=0.046). The LV mass and the values of the E/E' ratio did not show any association with the LA dimension (Table 3, middle panel).
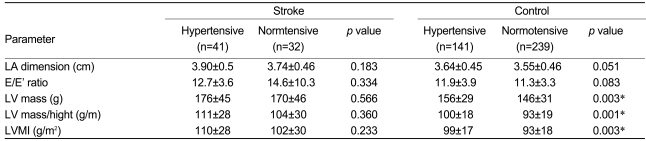
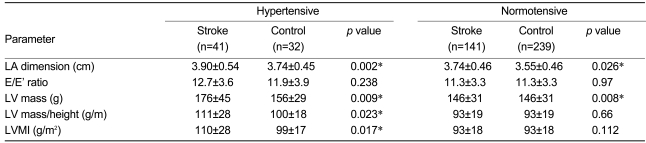
To estimate the effects of hypertension on the LA dimension, the subjects were further divided intotwo subgroups according to the presence or absence of a previous history of hypertension. For both the stroke and control groups, the LA dimension did not show a significant difference between subjects who did and did not have a history of hypertension (Table 4). However, the LA dimension of the stroke group was greater than that of the control group regardless of a history of hypertension (Table 5). In the absence of a history of hypertension, the LV mass for the stroke group was greater than that for the controls (170±46 g vs. 146±31 g, respectively; p=0.008); however, LV mass/height and the LV mass index of the stroke group were not greater than those of the controls. Univariate analysis of variance indicated that the LV mass index showed a closer association with LA dimension than with the LV mass or LV mass/height (corrected model F=11.114, p<0.0001, p<0.0001, p=0.879, and p=0.004, respectively). In cases with a history of hypertension, the LV mass, LV mass/height, and LV mass index of the stroke group were greater than those of the controls (176±45 g vs. 156±29 g, p=0.009; 111±28 vs. 100±18, p=0.023; and 110±28 vs. 99±17, p=0.017). Only the LV mass index, and not the LV mass or LV mass/height, showed an association with the LA dimension in the univariate analysis (corrected model F=17.311, p<0.0001, p=0.021, p=0.270, and p=0.131, respectively). Consequently, it was concluded that regardless of a history of hypertension, the LA dimension in the stroke group was greater than that in the control group, and the LV mass index had a positive relationship with the LA dimension in both the stroke and control groups.
Thus, the LA dimension was significantly associated with the LV mass index and stroke but not with hypertension.
Characteristics of the mitral LV inflow pattern in the stroke and control groups
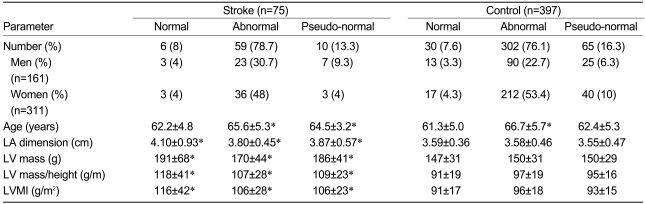
As our study population only included cases with preserved LVSF and sinus rhythm, impaired and abnormal relaxation patterns of mitral LV inflow were the most commonly observed patterns in both groups, with no cases showing the restrictive pattern. The incidences of each mitral inflow pattern were not different between the stroke and control groups, and the association between an abnormal relaxation pattern and advanced age is well known (Table 6). Regardless of the mitral inflow pattern, the LA dimension of the stroke group was significantly greater than that of the controls. The same results were observed for the LV mass, LV mass/height, and LV mass index. Univariate analysis indicated that there was no association between the mitral inflow pattern and either the LA dimension or stroke (F=1.122, p=0.327; F=0.218, p=0.804, respectively).
Relationship of stroke with LA dimension, age, and LV mass index
The above results indicate that the LA dimension has clear associations with age, stroke, and LV mass index, and a marginal relationship with history of hypertension, in a univariate analysis of variance. In a logistic regression analysis for stroke performed with these interrelated parameters, the LV mass index and LA dimension showed significant relationships with stroke (Odds ratio=1.021, 95% CI=1.007-1.035, p=0.003; and Odds ratio=2.081, 95% CI=1.106-3.917, p=0.023, respectively). Hypertension showed a marginal relationship with stroke (Odds ratio=0.581, 95% CI=0.338-0.999, p=0.050), and age was not related to stroke in the present study (Odds ratio=0.997, 95% CI=0.931-1.025, p=0.344) (Table 7).
DISCUSSION
Previous studies have identified a number of echocardiographic variables, including the LA dimension, as being of independent clinical value for predicting the likelihood of CVD events and overall mortality. However, with regard to the relationship between the LA dimension and stroke, most studies have been performed in heterogeneous populations or in subjects with established CVD such as myocardial infarction, abnormal LVEF, or atrial fibrillation. Although the Framingham Heart Study [11] had a prospective design and was able to determine the relationship between the LA dimension and stroke, the systemic hypertension criteria used were markedly different from those used in the present study. Previously, hypertension was considered to be present when the systolic pressure was above 160 mmHg or the diastolic pressure was above 95 mmHg. The Cardiovascular Health Study [12], which also reported a relationship between the LA dimension and stroke, was performed only in subjects older than 65 years. The Stroke Prevention in Atrial Fibrillation Study supported an association between stroke and LA enlargement [13], whereas two other cohort trials examining this issue did not show such a clear relationship, even after enrolling exclusively cases with atrial fibrillation [14,15]. Recently, Barnes et al. (3) reported that the LA dimension is related to the first ischemic stroke in people older than 65 years. However, the relationship between the LA dimension and stroke has not been investigated in detail in subjects with preserved LVSF and sinus rhythm and without overt CVD or in those of relatively young age. The present case-control study in such subjects showed that an increase in the LA dimension by itself has a clear relationship with stroke in subjects presenting with preserved LVSF and normal sinus rhythm; thus, neither an age older than 65 years or compromised LVSF with obvious CVD is a requirement for the relationship between the LA dimension and stroke.
Mechanism behind the relationship between stroke and the LA dimension
The mechanisms underlying the relationship between stroke and an enlarged LA are not completely understood. The most plausible hypothesis is that LA dilatation promotes stasis of blood, which in turn results in a propensity for thrombus formation and increased risk of embolism. The thrombogenicity of a dilated left atrium has been supported by the results of several transesophageal echocardiographic studies showing an association between an enlarged left atrium and spontaneous echocardiographic contrast [16-18].
Alternatively, the LA enlargement may be a surrogate marker representing an adaptive response to some variously caused endothelial dysfunction that affects the systemic vascular bed. Endothelial dysfunction results from the abnormal regulation of and response to many different cytokines and paracrine hormones, including nitric oxide, angiotensin II and its receptor, plasminogen nhibitor I, thrombomodulin, and endothelin [19]. In particular, angiotensin II and its receptor are well-known 30 The Korean Journal of Internal Medicine Vol. 24, No. 1, March 2009 enhancers of fibrosis and thrombogenicity, and can hinder myocardial electrical coupling in the atrium, resulting in atrial dilatation and thromboembolism [20,21]. In the Losartan Intervention for End Point Reduction in Hypertension study, losartan significantly decreased the incidence of stroke in comparison with atenolol [22].
Left atrial enlargement has frequently been associated with more severe cases of congestive heart failure, myocardial infarction, and systemic hypertension, and with chronic atrial fibrillation. Nevertheless, it is noteworthy that the relationship between stroke and LA dimension was also observed in the present study, in which the subjects had preserved LVSF and normal sinus rhythm without evidence of CVD. It may be that LA enlargement promotes the development of asymptomatic interim atrial fibrillation, which in turn places the patient at increased risk for stroke.
Relationship of LV mass index with LA dimension and stroke
Left atrial enlargement and left ventricular hypertrophy are manifestations of cardiac target organ damage in patients with established hypertension [23]. In an analysis of the Framingham Heart Study cohort, Bikkina et al. [24] reported that increased LV mass predicts stroke. Even after adjustment for LV mass index and systemic hypertension, the LA dimension still maintained its relationship with stroke in the present study. Furthermore, the relationship was also observed in cases without hypertension. These findings suggest that the relationship cannot be completely accounted for by LV mass and arterial blood pressure and that LA enlargement may serve as a surrogate marker for other unidentified risk factors for stroke.
Limitations and clinical implications
Echocardiographic examinations using the M-mode have a tendency to intrinsically miscalculate the LA dimension. In the present study, the LA dimension did not increase markedly according to the deterioration of the mitral inflow pattern, and the incidence of the pseudonormal pattern was greater than that reported previously [25]. Therefore, to resolve these issues, further studies that include the measurement of LA volume in a larger study population are required. The potential for bias was reduced by the routine assessment of echocardiograms in a blind manner with regard to subject characteristics. Even with the clear association between stroke and LA dimension demonstrated in the present case-control study, further prospective cohort investigations are needed to determine a cause and effect relationship.
The results of the present study indicated that the LA dimension was significantly related to the incidence of stroke. Although this relationship was somewhat attenuated after adjusting for LV mass index, it remained significant, and we suggest that strategies for the prevention of stroke in patients with sinus rhythm should be considered in cases with LA enlargement. It is also necessary to investigate the mechanism underlying the above relationship in order to improve the management of patients in such clinical conditions.