Bone Marrow T Cells are Superior to Splenic T Cells to Induce Chimeric Conversion After Non-Myeloablative Bone Marrow Transplantation
Article information
Abstract
Background/Aims
The bone marrow functions not only as the primary B-lymphocyte-producing organ but also as a secondary lymphoid organ for CD4 and CD8 cell responses and a site of preferential homing and persistence for memory T cells. Bone marrow T (BM-T) cells are distinguished from peripheral blood T cells by surface phenotype, cytokine secretion profile, and immune functions. In this study, we evaluated the alloreactive potential of donor lymphocyte infusion (DLI) using BM-T cells in mixed chimerism compared to that using spleen T (SP-T) cells.
Methods
Cells were prepared using established procedures. BM-T cells were obtained as a by-product of T-cell depletion in BM grafting and then cryopreserved for subsequent DLI. We performed DLI using BM-T cells in allogeneic mixed chimera mice on post-BMT day 21.
Results
When the same dose of T cells, 5-10×105 (Thy1.2+), fractionated from BM and spleen were administered into mixed chimeras, the BM-T group showed complete chimeric conversion, with self-limited graft-versus-host disease (GVHD) and no pathological changes. However, the SP-T group showed persistent mixed chimerism, with pathological signs of GVHD in the liver and intestine.
Conclusions
Our results suggest that DLI using BM-T cells, even in small numbers, is more potent at inducing chimeric conversion in mixed chimerism than DLI using SP-T cells. Further study is needed to determine whether cryopreserved BM-T cells are an effective cell source for DLI to consolidate donor-dominant chimerism in clinical practice without concerns about GVHD.
INTRODUCTION
Allogeneic bone marrow transplantation (ABMT) is a curative treatment for various hematological malignancies. The primary curative potential may be attributable to alloreactive donor T cells in the graft that mediate a graft-versus-leukemia (GVL) effect, rather than to the elimination of all malignant cells by myeloablative conditioning [1,2]. When some patients inevitably relapse despite aggressive treatment, donor lymphocyte infusion (DLI) following ABMT can be an effective alternative for inducing a second remission [3,4]. However, this beneficial effect of the DLI-mediated GVL effect, which originates from alloreactive donor T cells, is often counterbalanced by DLI-mediated graft-versus-host disease (GVHD) [5].
Recently, bone marrow T (BM-T) cells have attracted renewed interest because they have different surface phenotypes, subsets, and activation states than their peripheral counterparts [6]. Memory T cells undergo extensive migration from the blood to the BM and vice versa [7]. The BM plays an important role in preferential homing and extensive proliferation of memory T cells and contributes considerably to the long-lived memory T-cell pool [8-10]. Not only are BM-T cells more activated than their splenic counterparts, but they have a higher rate of local proliferation [11]. Although BM-T (NK1.1-CD4+ or CD8+) cells do not induce lethal GVHD, even in high numbers, they mediate vigorous graft-versus-tumor activity and facilitate engraftment of hematopoietic progenitor cells [6,12]. These studies suggest that BM-T cells rather than peripheral T cells are a useful cellular source for adoptive immunotherapy following ABMT.
Non-myeloablative bone marrow transplantation (NMT) and allogeneic mixed chimerism can provide an adequate environment for diminishing susceptibility to DLI-mediated GVHD and an immunological platform for DLI in mouse and human models [13,14]. In patients treated with DLI, a successful GVL effect is often associated with conversion to complete donor chimerism, which supports the concept of a graft-versus-host response as part of the GVL effect [15,16]. Thus, a quiet chimeric conversion following DLI is desirable for reaching an optimal DLI-mediated GVL effect without the occurrence of GVHD. In a mouse model, the administration of non-tolerant donor spleen cells to established mixed chimeras converted mixed hematopoietic chimerism to full donor chimerism without the concomitant development of GVHD; however, DLI in humans frequently results in serious GVHD and life-threatening complications [17]. Yet the use of BM-T cells as the DLI source has received less attention than the use of spleen T (SP-T) cells in allogeneic mixed chimerism prepared with NMT.
In this study, we evaluated the beneficial alloreactivity of DLI using cryopreserved BM-T cells to effectively induce chimeric conversion without the occurrence of GVHD in major histocompatibility complex (MHC)-mismatched NMT. The BM-T cells were a by-product of the T-cell depletion (TCD) procedure in BM grafting.
METHODS
Mice
Female BALB/c (H-2kd) and C57BL/6 (B6; H-2kb) mice, 8-10 weeks old, were purchased from Samtago (Kwangju, Korea). The mice were maintained under specific pathogen-free conditions in an animal facility with controlled humidity (55±5%), light (12/12 hours light/dark), and temperature (22±1℃). The air in the facility was passed through a HEPA filter system that was designed to exclude bacteria and viruses. Animals were fed mouse chow and tap water ad libitum. The protocols used in this study were approved by the Animal Care and Use Committee of The Catholic University of Korea.
Preparation of mixed chimera
Allogeneic mixed chimeras were prepared using established procedures [18,19]. Briefly, 1 day before BMT, recipient mice (BALB/C, H-2Kd) were injected intraperitoneally with 200 µL PBS that contained 40 µL reconstituted anti-asialoganglioside GM1 (anti-ASGM1; Wako Chemicals, Osaka, Japan). Recipient mice were exposed to a single dose of 500 cGy X-rays from a Mevatron MXE-2 (Siemens Co., New York, NY, USA) with a focus-to-skin distance of 100 cm and a rate of 200 cGy/min. Donor BM cells were collected into Cedarlane cytotoxicity medium (RPMI 1640 medium supplemented with 25 mM HEPES buffer and 0.3% bovine serum albumin) by flushing the shafts of the femurs and tibias of C57BL/6 mice. Resuspended BM cells were depleted of T cells by incubation with anti-Thy-1.2 micro-beads (Miltenyi Biotec, Auburn, CA, USA) according to the manufacturer's protocol. Within 6 hours of irradiation, 2×107 T-cell-depleted BM cells in a final volume of 0.2 mL PBS were reinfused into recipient mice.
Cell cryopreservation and thawing
We suspended the collected BM-T cells in 1 mL serum-free cell freezing medium (BAMBANKER™; Wako Chemicals). The cells were placed in cryotubes and stored in a chamber overnight at -80℃. The cells were frozen at -80℃ and subsequently preserved in liquid nitrogen. The samples were rapidly thawed in a water bath at 37℃. The freezing medium was removed by centrifugation.
DLI
Mononuclear cells from spleen and BM of C57BL/6 mice (donors) were incubated with anti-CD4, anti-CD8, and anti-Thy1.2 micro-beads (Miltenyi Biotec) at 4℃ for 15 minutes. After washing with MACS buffer (1% BSA, 2 mM EDTA in PBS, pH 7.4), the cells were subjected to positive selection through magnetic cell sorting separation columns. On day 21 after BMT, chimeric mice were infused via the tail vein with donor-type CD4+, CD8+, unfractionated spleen cells, and cryopreserved BM-T (Thy1.2+) cells.
Flow cytometric analysis
Donor (H-2Kb) and recipient (H-2Kd) cells were distinguished during lymphoid gating by staining with fluorescein isothiocyanate (FITC)-labeled anti-H-2Kb and phycoerythrin-labeled anti-H-2Kd antibodies (Pharmingen, San Diego, CA, USA), respectively. To analyze the degree of donor chimerism in the peripheral blood, we performed lineage-specific staining using antibodies to T-cell marker CD4, CD8, myeloid maker CD11b, and B-cell marker B220 (all from Pharmingen). Stained cells were analyzed using CellQuest software and a FACSCalibur flow cytometer (both from Becton Dickinson, Mountain View, CA, USA). Percentages of donor-derived cells were calculated by dividing the percentage of donor cells by the total net percentage of donor plus recipient cells that showed positive staining for lineage-specific markers [18]. SP-T cells and BM-T cells used in DLI were characterized by staining with FITC-anti-TCRβ, PE-anti-NK1.1, PerCP-anti-CD4, and APC-anti-CD8 (all purchased from Pharmingen).
Histopathological analysis of GVHD
Survival after BMT was monitored daily, and the degree of clinical GVHD was assessed weekly using a scoring system that summed changes in five clinical parameters: weight loss, posture, activity, fur texture, and skin integrity [20]. Mice were killed at days 21 and 42 after DLI for blinded histopathological analysis of GVHD target organs (small and large intestine, liver, and stomach). Organs were harvested, cryo-embedded, and subsequently sectioned. Tissue sections were fixed in 10% buffered formalin and stained with hematoxylin and eosin for histological examination.
Mixed lymphocyte reaction
Spleen cells were used as both responders (BM-T cells and SP-T cells of mixed chimeric or donor mice) and stimulators (whole spleen cells of mixed chimeras, irradiated with 2500 cGy) in this assay [18]. Spleen cells were removed using ACK lysis buffer, washed, and resuspended in complete culture medium (RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 1 mM sodium pyruvate, 5×105 M 2-ME, 20 mM HEPES, 100 U/mL penicillin, and 100 µg/mL streptomycin). Aliquots of 4×105 responder splenocytes were cultured with 4×105 irradiated (2,000 cGy) stimulator splenocytes in 96-well, round-bottomed microwell trays (Corning Inc., Corning, NY, USA). Each well, containing 200 µL complete medium at 37℃ in a humidified 5% CO2 atmosphere, was pulsed with 1 µCi [3H]TdR (NEN Life Science Products Inc., Boston, MA, USA) after incubation for 3.5 days and was harvested 6 h later using an automated harvester (PHD Cell Harvester; Cambridge Technology, Inc., Cambridge, MA, USA). Results are expressed as the mean±SD cpm of triplicate samples. The stimulation index was calculated by comparing the anti-stimulator response with the anti-self response.
Statistical analysis
Comparison of numerical data between the two groups was performed with the non-parametric Mann-Whitney test. Statistical analysis was performed using SPSS 10.0 for Windows (SPSS Inc., Chicago, IL, USA). P<0.05 was considered significant. Data are presented as the mean±SD.
RESULTS
Induction of allogeneic mixed chimerism in BALB/c mice treated with T-cell-depleted NMT
Inhibition of natural killer (NK) cell activity abolishes the rejection of T-cell-depleted BM grafts in a fully MHC-mismatched NMT setting. In the present study, we applied the same protocol to the induction of allogeneic mixed chimerism between host (BALB/c, H-2kd) and donor (C57BL/6, H-2kb) mice. Multicolor flow cytometric analysis was performed on peripheral lymphocytes to determine whether T-cell-depleted NMT induced allogeneic mixed chimerism in the host. A representative example of multi-lineage mixed chimerism in the host at 3 weeks after T-cell-depleted NMT is shown in Fig. 1A.

Multicolor flow cytometric analysis for multilineage mixed chimerism in mixed chimera. (A) Mixed chimera (C57BL/6 → BALB/C) was prepared by total body irradiation at a dose of 500 cGy following treatment with anti-ASGM1 on day 1 of T-cell-depleted NMT. The state of allogeneic mixed chimerism was evaluated at 3 weeks after BMT in the peripheral blood. Donor (H-2kb) and recipient (H-2kd) cells were distinguished in lymphoid gating by staining with FITC-anti-H-2kb and PE-anti-H-2kd. Lineage-specific staining was performed with antibodies to T-cell markers (Percp-anti-CD4, APC-anti-CD8), myeloid marker (Perp-anti-CD11b), and B-cell marker (APC-anti-B220). Percentages of donor-derived cells were calculated by dividing the percentages of donor cells by the total net percentage of donor plus recipient cells stained positively for lineage-specific makers; they are expressed in the upper right corner of each plot. One representative of 12 measured mixed chimeras is shown (B). The percentage of donor-derived cells stained positive for H-2kd (total lymphocytes), CD4, CD8, CD11b, and B220. Values are the mean from three independent experiments, and bars show the means±SD.
We confirmed successful induction of allogeneic mixed chimerism through expression of both H-2kb and H-2kd in the host. The mean±SD relative percentages of cells of donor origin in the peripheral blood were 77.4±10.3% of total lymphocytes, 20.0±13.3% of CD4+ T cells, 77.4±12.5% of CD8+ T cells, 80.4±15.1% of CD11b+ cells, and 99.4±0.3% of B220+ B cells (Fig. 1B).
Three weeks post-BMT conversion of mixed to complete donor chimerism following DLI using T-cell subsets of CD4+, CD8+, whole spleen cells, and BM-T (Thy1.2+) cells
In the early post-transplant period, at day 21, we performed DLI using 2×106 purified CD4+, 1×106 purified CD8+, and 1×107 unfractionated spleen cells in allogeneic mixed chimera mice because, in general, more than 1×107 spleen cells have been used as the cell dose and source for DLI (they contain about 20% of CD4+ and 10% of CD8+ T cells). Additionally, we performed DLI using 5×105 cryopreserved BM-T (Thy1.2+) cells, because we planned to perform DLI using BM-T cells obtained and cryopreserved during the TCD procedure of BM grafting. According to our preliminary data, we confirmed the percentage of T-cell-depleted (Thy1.2-) cells and BM-T (Thy1.2+) cells; the former included about 95% and the latter about 5% of the total BM cells. If in BM grafting 2×107 T-cell-depleted BM cells are used, we would expect there to be about 1×106 BM-T (Thy1.2+) cells, which would be reduced by about half, to about 5×105, by cryopreservation and thawing. In all experiments, the purity of isolated BM-T and SP-T cells was >90%. Expression of lineage-specific markers, such as TCRβ+, NK1.1, CD4, and CD8, was investigated in BM-T and SP-T cells (Fig. 2A).

Changes in donor chimerism following DLI using a half cell dose of cryopreserved BM-T (Thy1.2+) cells, a by-product of the TCD procedure in BM grafting, to mixed chimeras on day 21 after NMT. (A) Surface phenotype and purity of the cellular sources for DLI obtained from C57BL/6 donor mice. DLI using 1×107 whole spleen cells, 2×106 CD4+ spleen cells, 1×106 CD8+ spleen cells, and 5×105 BM-T (Thy1.2+) cells was conducted by intravenous infusion on day 21 posttrans-plantation. Spleen and BM cells were harvested from donor mice. Resuspended mononuclear cells were incubated with anti-mouse CD4-, CD8- and Thy-1.2-coated magnetic beads and subjected to positive selection using MACS columns. Cellular sources of DLI were characterized by staining with FITC-anti-TCRβ, PE-anti-NK1.1, PerCP-anti-CD4, and APC-anti-CD8. The purity of the CD4+ and CD8+ spleen cells or the Thy1.2+ BM cells used for DLI was analyzed by flow cytometry to verify 80-99% purity. (B) Changes in donor chimerism in the peripheral blood following DLI given to mixed chimeras on day 21 post-BMT. Recipients received DLI with whole spleen cells (◆, n=7), CD4+ spleen cells (■, n=7), CD8+ spleen cells (▲, n=12), BM Thy1.2+ T cells (×, n=8), or total body irradiation alone (○, n=8) on day 21 after BMT. After 3 weeks, the peripheral blood of DLI recipients was stained with FITC-anti-H-2kb and PE-anti-H-2kd. (C) Changes in body weight following DLI. All recipients of DLI showed similar changes except the CD4+ SP-T cell group, which showed severe clinical GVHD symptoms such as serious weight loss, decreased activity, hunched posture, diarrhea, and scaled skin on the tail.
The degree of chimeric conversion was evaluated in the peripheral blood by multicolor flow cytometry on day 21 after DLI. When unfractionated spleen cells, CD8+ spleen cells, and BM-T (Thy1.2+) cells were used for DLI, the host converted to full donor chimerism without DLI-mediated GVHD symptoms. In contrast, mice that received DLI using CD4+ spleen cells showed not only a persistent mixed chimeric state but also various serious DLI-mediated GVHD symptoms, such as hunched posture, alopecia, weight loss, diarrhea, and epidermal scaling on the tail (Fig. 2B and 2C).
BM-T (Thy1.2+) cells in DLI are more potent than SP-T (Thy1.2+) cells at inducing conversion of mixed to full donor chimerism on day 21
The above results suggested that BM-T cells potently induced chimeric conversion despite the small dose. Thus, we next compared the effect of DLI using BM-T and SP-T cells at the same cell dose in allogeneic mixed chimerism. DLI with BM-T cells administered on day 21 led to conversion to full donor chimerism. The degree of chimeric conversion was evaluated in the peripheral blood by multicolor flow cytometry. The results showed that mixed chimeric mice in the group that received DLI using 5×105 BM-T cells showed high-level donor chimerism in the lymphoid compartment. The percentages of cells of donor origin in the peripheral blood were 98.2±0.9% of total lymphocytes, 88.0±2.7% of CD4+ cells, and 95.6±0.7% of CD8+ cells. In contrast, mice in the group receiving DLI using the same dose of SP-T cells showed a lower degree of donor chimerism in the peripheral blood: 59.2±47.5% of total lymphocytes, 45.2±43.8% of CD4+ cells, and 53.5±49.4% of CD8+ cells. When the cell dose of DLI was doubled to 1×106 BM-T or SP-T cells, DLI using BM-T cells increased the degree of donor chimerism, but that using SP-T cells did not. The percentages of cells of donor origin in mice that received DLI using BM-T cells were 97.8±1.2% of total lymphocytes, 83.7±1.6% of CD4+ cells, and 97.0±0.0% of CD8+ cells on day 21 (Fig. 3A) and 99.8±0.1% of total lymphocytes, 99.3±0.6% of CD4+ cells, and 99.3±0.4% of CD8+ cells on day 42. However, the percentages of cells of donor origin in mice that received DLI using SP-T cells were 59.7±45.4% of total lymphocytes, 32.5±44.7% of CD4+ cells, and 56.9±48.6% of CD8+ cells on day 21 and 46.3±56.3% of total lymphocytes, 30.6±40.0% of CD4+ cells, and 48.8±62.2% of CD8+ cells on day 42 (Fig. 3B). These results demonstrate that DLI using BM-T cells was superior to that using SP-T cells at inducing chimeric conversion following NMT.
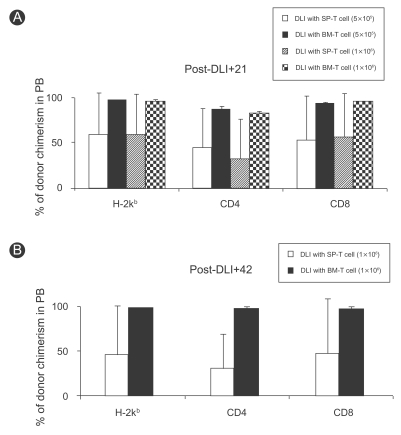
Comparison of chimeric conversion ability between BM-T (Thy1.2+) and SP-T (Thy1.2+) cells. Multicolor flow cytometric analysis for conversion from mixed to full donor chimerism following DLI comparing BM-T (Thy1.2+) and SPT (Thy1.2+) cells at the same cell dose. Multi-lineage donor chimerism in the peripheral blood was characterized by lineage-specific markers using FITC-anti-H-2kb, PE-anti-H-2kd, Percp-anti-CD4, and APC-anti-CD8. Percentages of donor chimerism were calculated by dividing the percentage of donor cells (H-2kb) by the total net percentage of donor plus recipient cells (H-2kd) stained positively for lineage-specific markers. (A) Mixed chimeric mice received DLI with BM-T or SP-T cells of 5×105 (n=6) and 1×106 (n=6) at day 21 post-BMT. Changes in donor chimerism were evaluated on day 21 after DLI. (B) Donor chimerism levels in mice that received DLI with 1×106 BM-T and SP-T cells were again evaluated on day 42 after DLI. Values are the mean±SD from three independent experiments.
Donor BM-T (Thy1.2+) cells facilitate conversion to full donor chimerism without GVHD, but donor SP-T (Thy1.2+) cells do not
GVHD in transplant recipients is caused by total body irradiation-conditioning procedures and donor T-cell attack on recipient epithelial tissues such as the gut, liver, and skin. Many patients die because of GVHD. Thus, assessment of clinical GVHD is important after BMT. We measured the body weight of BMT recipients three times every week. The body weight in DLI recipients (BM-T and SP-T cell groups) was almost unchanged. The body weight of mice that received DLI using BM-T cells decreased slightly but soon recovered. We believe that loss of body weight is a passing phenomenon in engraftment of the BM graft (Fig. 4A). We also observed posture, mobility, skin, and fur and made histopathological assessments of the stomach, intestine, and liver by hematoxylin and eosin staining on day 21 after DLI. We observed no tissue damage or pathological changes in DLI recipients injected with BM-T cells. In contrast, mice that received SP-T cells showed lymphocytic infiltration in the periportal area and tissue damage in the liver and intestine (Fig. 4B). These results indicate that BM-T cells prevented GVHD development and that SP-T cells induced severe GVHD in our DLI models.
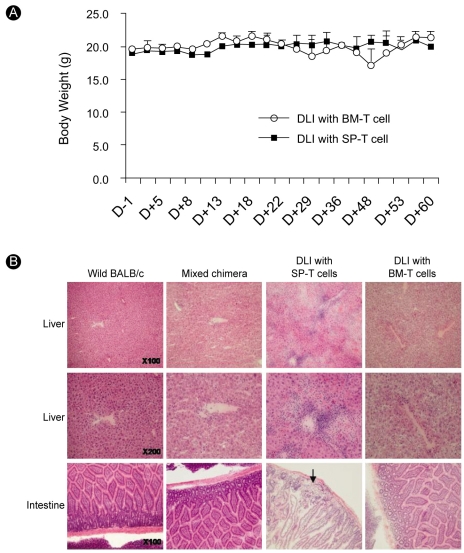
Evaluation of GVHD following DLI using BM-T (Thy1.2+) and SP-T (Thy1.2+) cells. Mononuclear cells harvested from spleen and BM of C57BL/6 mice (donors) were incubated with anti-Thy1.2 micro-beads and positively selected through MACS columns. DLI with the same dose of 5×105 BM-T or SP-T cells was performed on day 21 following BMT. (A) Changes in body weight of recipients over a period of 9 weeks after BMT. No significant difference was detected between the BM-T (○, n=6) and SP-T (■, n=6) groups. (B) Histopathological changes in liver and intestine following DLI using BM-T and SP-T cells. Mice were killed on day 21 after DLI, and tissues were analyzed after hematoxylin and eosin staining. Tissue samples from liver and intestine are shown. Mixed chimeric mice showed histological findings identical to those of wild-type BALB/c mice. The SP-T group showed marked portal lymphoid infiltration, with necrosis in the liver and lymphoid infiltration with glandular loss in the intestinal mucosa (arrow), whereas the BM-T group showed only slight lymphoid infiltration in the portal area and no change in the intestinal mucosa.
In vitro evidence for ongoing alloreactivity of BM-T (Thy1.2+) cells in chimeric mice
To investigate the ability of BM-T cells to mount alloreactivity after infusion into mixed chimeric mice, we tested the capacity of BM-T cells to mount a proliferative response in vitro in a standard mixed lymphocyte reaction. The proliferative response of BM-T cells was higher than that of SP-T cells (mean±SD SI: 2.19±0.1 versus 1±0.4, p<0.05) of donor origin in mixed chimeric mice. Similarly, the response of BM-T cells was higher than that of SP-T cells (mean±SD SI: 3.15±0.1 versus 1.18±0.3, p<0.05) of mixed chimeric mice. This result suggests that BM-T cells had high alloreactivity but did not develop GVHD. This alloreactivity was apparently effective in chimeric conversion but did not induce GVHD (Fig. 5).

Proliferative response of BM-T (Thy1.2+) and SP-T (Thy1.2+) cells against spleen cells of mixed chimera mice. Mononuclear cells harvested from spleen and BM of wild-type C57BL/6 mice (donors) and mixed chimera mice on day 21 after BMT were incubated with anti-Thy1.2 micro-beads and positively selected through MACS columns. BM-T and SP-T cells (4×105 cells/well) were cultured with irradiated whole spleen cells (4×105 cells/well) from mixed chimeric mice in 96-well, U-bottomed plates. Each well was pulsed with 1 µCi [3H]TdR, incubated for 3.5 days, and harvested 6 h later with an automated harvester (PHD Cell Harvester; Cambridge Technology, Inc., Cambridge, MA, USA). Stimulation indexes were derived by responder anti-stimulator/responder anti-self. Results are expressed as SI, and p values were determined by non-parametric Mann-Whitney test.
Characteristics of BM-T (Thy1.2+) and SP-T (Thy1.2+) cells
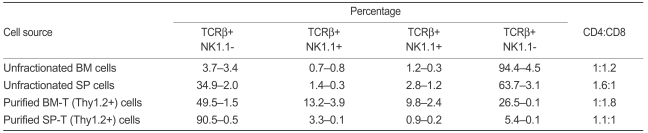
Although several studies have investigated T cells in BM and spleen, differences in function and characteristics between BM-T and SP-T cells have not been defined. Clarification of the biological characteristics of BM-T and SP-T cells is important because it may explain the mechanism of chimeric conversion through BM-T cells. Therefore, we analyzed the percentages of immune cells (i.e., T cells, NKT cells [NKT cells], NK cells) in BM and spleen using well-known fluorescence-conjugated phenotypic markers. The expression of specific surface markers of isolated T cells from BM and spleen was evaluated by flow cytometry. The CD4/CD8 ratios of BMT and SP-T cells were 20.1±1.8%: 37.0±2.1% and 48.6±0.5%: 44.3±1.4%, respectively. BM-T cells contained a high percentage of CD8 T cells. In particular, the percentages of TCRβ+NK1.1+ cells (13.2±3.9%) and TCRβ-NK1.1+ cells (9.8±2.4%) in BM-T cells were higher than those in SP-T cells (3.3±0.1% and 0.9±0.2%, respectively, Table 1). However, these approaches have not been sufficiently well developed to be generally accepted, as demonstrated by the unresolved problems of the correct function of NKTs and subpopulations of T cells.
DISCUSSION
Although BM is a primary lymphoid organ for the development of mature B cells and precursor T cells, its role in mature T-cell responses has often been neglected. Recently, the biological characteristics of BM-T cells were distinguished from those of blood T cells by their unique patterns of surface receptors, cytokine secretion, and immune functions [6]. First, the makeup of T-cell subsets in BM differs from that in peripheral T cells, and BM-T cells contain an unusually high proportion of NKT cells (15-50% of the BM TCR αβ+ cells), although TCR αβ+ cells constitute 3-8% of nucleated BM cells in humans and mice [21,22]. BM NK1.1-T cells do not induce GVHD, but those in the blood can induce lethal GVHD [23]. Second, long after priming, memory CD8 cells proliferate more extensively in the BM than they do in either secondary lymphoid or extra-lymphoid organs [8,9]. The CD4:CD8 ratio in the BM is about -1:2, which is inverted in comparison to the ratio found in peripheral lymph nodes and the blood, which ranges from -2:1 to -2.5:10 [6,22]. CD8+ T cells in donor BM grafts have the unique capacity to facilitate complete chimerism and eliminate tumor cells without non-lymphohematopoietic tissue injury [12,24]. Third, BM-T cell populations contain a high proportion of cells that display a memory phenotype that expresses low levels of CD45RA in humans [10] and high levels of CD44 in mice, because memory T cells migrate to the BM after priming [9,25,26]. BM memory CD8+ cells are more activated that their splenic counterparts and have a higher rate of local proliferation [8,11].
Hematopoietic stem cells reside in the peripheral blood following mobilization by the administration of granulocyte colony-stimulating factor, as well as in the BM. Recently, peripheral blood stem cell (PBSC) grafting has been used instead of BM grafting [27]. In earlier reports, PBSC transplantation showed rapid hematological recovery, lower transplantation-related mortality, and higher leukemia-free survival rates without increased risk of GVHD compared to BMT [28]. However, more recent reports have suggested worse outcomes and more chronic GVHD with PBSC than BM in HLA-matched sibling donor transplants in young patients [29-31]. These clinical observations suggest that these outcomes may result primarily from the different biological characteristics of T cells within PBSC and BM grafts. In the present study, BM-T (Thy1.2+) cells in DLI were more potent than SP-T (Thy1.2+) cells at inducing the conversion of mixed to full donor chimerism on day 21. Additionally, donor BM-T (Thy1.2+) cells facilitated conversion to full donor chimerism without GVHD, but donor SP-T (Thy1.2+) cells did not affect the degree of donor chimerism despite evidence of pathological changes consistent with GVHD. These results suggest that BM-T cells within BM grafts or DLI are superior to SP-T cells within PBSC grafts or DLI at safely inducing beneficial alloreactivity and the graft-versus-host reaction.
Although we did not precisely clarify the immunological mechanism of the more favorable clinical outcome after DLI using BM-T cells compared with SP-T cells, we speculate that several cell types might be potential candidates as key effector cells for complete chimeric conversion without the occurrence of GVHD. First, donor-derived NK-cell-based DLI was used safely to facilitate engraftment and induce graft-versus-tumor effects, because alloreactive NK cells have not been implicated in GVHD [22,33,34]. Our study shows that NK cells in purified BM-T (Thy1.2+) cells are about 10-fold higher than in purified SP-T (Thy1.2+) cells. Second, CD8+ T cells in the donor BM had a unique ability to eliminate normal and malignant host lymphohematopoietic cells without causing GVHD in nonmyeloablated hosts [12]. In contrast, donor CD8+ T cells from tissue sources outside the BM (lymph nodes and spleen) are capable of eliminating lymphohe-matopoietic tumors and facilitating stem cell engraftment in myeloablated hosts, but lethal GVHD is observed as the dose of purified CD8+ T cells is increased to achieve uniform tumor elimination [35,36]. Third, CD4+CD25+ Foxp3+ regulatory T (Treg) cells are more numerous in BM than in the thymus, lymph nodes, or blood, and BM CD4+CD25+ Foxp3+T cells are functional Tregs that mediate suppressive effects by inhibiting T cells. BM is an important site for T cell priming, migration or selective retention, function of Tregs, and harboring of antigen-specific memory T cells [37].
NMT leads to an initial state of lymphohematopoietic mixed chimerism, defined as the coexistence of donor- and recipient-derived lymphohematopoiesis. Allogeneic mixed chimerism can provide an immunological platform for adoptive immunotherapy with DLI for patients with malignant disease, as host T cells can resist GVHD mediated by DLI. In addition, the induction of mixed chimerism followed by delayed DLI provides an approach for inhibiting GVHD that optimizes GVL effects [15]. For this purpose, DLI may be an effective therapeutic intervention for patients with labile engraftment or with various hematological malignancies that have relapsed after BMT [17]. In the present study, during the TCD procedure in BM grafting, BM-T (Thy1.2+) cells, a by-product, could be obtained and then cryopreserved for DLI in the early post-BMT period. Cryopreserved BM-T cells, even in small numbers, provided sufficient allore-activity to induce complete chimeric conversion in allo-geneic mixed chimeras. Based on our results, we cautiously suggest that a small number of cryopreserved BM-T cells, a surplus obtained during BM harvesting, may be used effectively to consolidate donor-dominant chimerism or induce a GVL effect without concerns of GVHD following NMT.
In summary, we have demonstrated that compared to peripheral T cells, BM-T cells, even in small doses, have a more potent ability to induce chimeric conversion without the occurrence of GVHD despite a DLI operation in the early stages after transplantation. Additionally, our study suggest that DLI using BM cells might be used instead of DLI using peripheral lymphocytes to induce a beneficial alloreactive T cell response, such as chimeric conversion in clinical practice. Further clinical studies are needed to develop this concept from animal models to clinical practice.
Notes
This study was supported by a grant from the Korea Science and Engineering Foundation (R11-2002-098-08004-0) and the Catholic Institute of Cell Therapy Basic Science Programs Foundation made in the 2007 program year.