Clinical Meaning of Early Oxygenation Improvement in Severe Acute Respiratory Distress Syndrome under Prolonged Prone Positioning
Article information
Abstract
Background/Aims
Ventilating patients with acute respiratory distress syndrome (ARDS) in the prone position has been shown to improve arterial oxygenation, but prolonged prone positioning frequently requires continuous deep sedation, which may be harmful to patients. We evaluated the meaning of early gas exchange in patients with severe ARDS under prolonged (≥ 12 hours) prone positioning.
Methods
We retrospectively studied 96 patients (mean age, 60.1 ± 15.6 years; 75% men) with severe ARDS (PaO2/FiO2 ≤ 150 mmHg) admitted to a medical intensive care unit (MICU). The terms "PaO2 response" and "PaCO2 response" represented responses that resulted in increases in the PaO2/FiO2 ratio of ≥ 20 mmHg and decreases in PaCO2 of ≥ 1 mmHg, respectively, 8 to 12 hours after first placement in the prone position.
Results
The mean duration of prone positioning was 78.5 ± 61.2 hours, and the 28-day mortality rate after MICU admission was 56.3%. No significant difference in clinical characteristics was observed between PaO2 and PaCO2 responders and non-responders. The PaO2 responders after prone positioning showed an improved 28-day outcome, compared with non-responders by Kaplan-Meier survival estimates (p < 0.05 by the log-rank test), but the PaCO2 responders did not.
Conclusions
Our results suggest that the early oxygenation improvement after prone positioning might be associated with an improved 28-day outcome and may be an indicator to maintain prolonged prone positioning in patients with severe ARDS.
INTRODUCTION
Present treatment of acute respiratory distress syndrome (ARDS) is largely supportive in nature, and unacceptable levels of morbidity and mortality persist [1]. Traditionally, the definition of ARDS includes bilateral infiltrates on simple chest radiographs [2], but computed tomography scanning has highlighted the patchy, heterogeneous nature of the disease. In particular, the dependent lung regions are often severely affected by alveolar flooding, increased surface tension, and collapse [3]. Gravitational effects directing pulmonary blood flow augment perfusion to these regions, thereby worsening shunt fraction [4].
The prone position was reported as early as the mid-1970s to improve oxygenation in patients with hypoxic respiratory failure [5], and many investigators have reported that ventilation of ARDS patients in the prone position improves arterial oxygenation [6-13]. Recently, several studies have suggested that prone positioning, when initiated early and applied for most of the day, may reduce mortality in patients with severe ARDS [14,15]. However, prolonged prone positioning frequently requires continuous sedation of the patient, which has been reported to adversely affect critically ill patients [16,17]. Thus, if we could predict the effect of prolonged prone positioning in the early stages of the prone position, it may obviate the unnecessarily heavy sedation associated with prone positioning.
There are debatable reports as to whether improvements in gas exchange in response to the prone position are actually associated with an enhanced outcome; however, these studies had methodological differences and patients were positioned for only a few hours each day [18,19]. We thus hypothesized that an early gas exchange response to first placement in the prone position might be associated with survival in ARDS patients, as reported previously [18], especially when the time spent prone is prolonged. Moreover, few studies have evaluated patients with severe ARDS who underwent prolonged prone positioning.
Our aim was to evaluate clinical characteristics, gas exchange responses after first placement in the prone position, outcomes, and the clinical meaning of early gas exchange in patients with severe ARDS under conditions of prolonged prone positioning (≥ 12 hours, whether clinical status improved or not) in a university-affiliated tertiary care hospital.
METHODS
Subjects
This study was performed in a 28-bed medical intensive care unit (MICU) at the Asan Medical Center, a tertiary referral academic care hospital in Seoul, Korea (about 2,200 beds). The inclusion period was from January 1, 2000 to July 31, 2006. We conducted a retrospective search of all adult patients (≥ 18 years of age) admitted to the MICU. The inclusion criterion was severe ARDS (PaO2/FiO2 ≤ 150 mmHg with a positive end-expiratory pressure (PEEP) of at least 8 cm H2O, and bilateral chest radiography showing lung infiltrate without evidence of cardiac failure). We enrolled a total of 96 adult patients who were diagnosed with severe ARDS and who were placed in the prone position for ≥ 12 hours in the MICU until the patient's position was changed from prone to supine due to improvement (PaO2/FiO2 ≥ 150 or FiO2 requirement ≤ 0.5 at PEEP of 8 cm H2O or lower, and an improved chest radiography finding) or deterioration (with no improvement in gas exchange after prone positioning).
Our study protocol regarding medical record review was approved by the Institutional Review Board of the Asan Medical Center. We confirmed that prone positioning was performed after obtaining informed consent from each patient's surrogate, identified through medical records.
Data collection
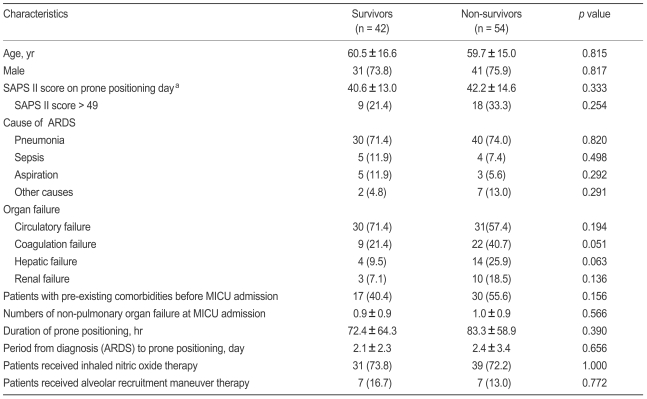
The following data were gathered from the medical records of each patient: age, gender, causes of ARDS, preexisting co-morbidities, numbers of non-pulmonary organ failures when diagnosed with ARDS, length of stay in the MICU and in the hospital, and complications attributable to prone positioning. The primary end-point was 28-day mortality. On the day of prone positioning, we calculated the Simplified Acute Physiology Score (SAPS) II as an index of illness severity [20] and recorded biochemical variables indicative of organ failure (renal, hepatic, circulatory, and coagulation failure) as defined by the Acute Respiratory Distress Syndrome Network trial of the US National Heart, Lung, and Blood Institute [21]. Data on adjuvant therapies such as inhaled nitric oxide (NO) and/or alveolar recruitment maneuver (ARM) therapy were also collected [22].
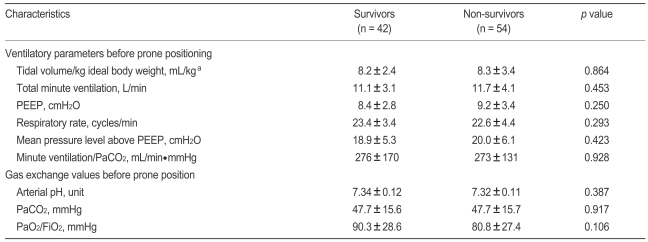
Arterial blood gas exchange variables (PaO2, PaCO2, pH) and ventilator variables (tidal volume in the pressure-controlled mode, level of pressure support, PEEP, and FiO2) were collected just before and around 8 hours after first placement in the prone position. If arterial blood gas analysis (ABGA) was not checked at 8 hours after prone positioning, we collected ABGA data until 12 hours after prone positioning. Of these variables, we categorized whether arterial pH was above pH 7.25 in keeping with some experts' recommendation about mechanical ventilation in patients with ARDS [23]. Gas exchange responses to placement in the first prone position were defined as follows: "PaO2 response" and "PaCO2 response" were defined as an increase in the PaO2/FiO2 ≥ 20 mmHg and as a decrease in PaCO2 ≥ 1 mmHg, respectively, after 8 to 12 hours in the prone position [18].
Statistical analysis
Continuous variables are expressed as means ± SD. The Student's t-test was used to compare continuous variables, and the chi-square or Fisher's exact tests (for small numbers) were used to compare categorical variables. Patient survival was analyzed using the Kaplan-Meier method, and compared using the log-rank test. Factors with p values less than 0.05 or equal by univariate analysis were entered into the model. Statistical analyses were performed using the SPSS version 14.0 (SPSS Inc., Chicago, IL, USA). A two-tailed p value less than 0.05 was deemed to indicate statistical significance.
RESULTS
Clinical characteristics of patients
In all enrolled patients (mean age, 60.1 ± 15.6 years; 75% men), the mean duration of prone positioning was 78.5 ± 61.2 hours, the mean period from diagnosis (ARDS) to prone positioning was 2.3 ± 2.9 days, SAPS II score at time of prone positioning was 42.2 ± 13.9, and the lengths of MICU and hospital stays were 21.7 ± 18.5 and 35.2 ± 32.8 days, respectively. Pulmonary ARDS was the most common cause of initial MICU admissions (72.9%). The 28-day mortality rate after MICU admission was 56.3%. All subjects required sedative agents. A neuromuscular blocking agent was administrated to 86 patients (89.6%) during prone positioning, and the total duration of neuromuscular blockade use was 6.2 ± 5.7 days. Complications attributable to prone positioning were skin abrasion (three patients) and unintended extubation (one patient).
No statistically significant difference was found between survivors and nonsurvivors in terms of clinical characteristics, arterial blood gas exchange variables, or ventilator variables just before prone positioning (Tables 1 and 2). When all enrolled patients were stratified by level of illness severity (SAPS II score ≤ 49 vs. > 49 on the day of prone positioning) [13], no significant difference was observed. Of all enrolled subjects, 72.9% and 14.6% patients had received inhaled NO and ARM therapy, respectively, but no outcome difference was associated with the inhaled NO and/or ARM trial (Table 1).
Early gas exchange responses after prone positioning and clinical outcomes
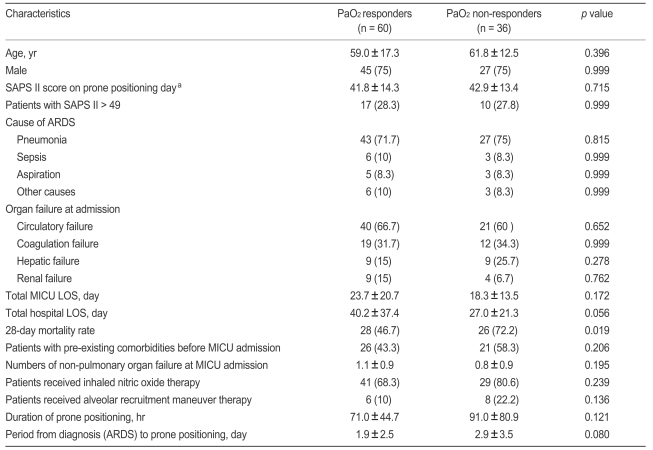
After prone positioning, we found 60 (62.5%) and 51 (53.1%) patients who met our definitions as PaO2 and PaCO2 responders. For all PaO2 responders, the mean PaO2/FiO2 increase at 8 to 12 hours of prone positioning was 75.4 ± 47.2 mmHg (median, 64.3; range, 20 to 215) and for all PaCO2 responders, the mean PaCO2 change was - 10.6 ± 10.3 mmHg (median, - 7.4; range, - 42 to 1).
We found that the PaO2 response, but not the PaCO2 response, was associated with an improved 28-day outcome (Tables 3 and 4). The Kaplan-Meier estimate of survival at 28 days showed the same result (Fig. 1A and 1B). When we classified four subgroups from the PaO2 and PaCO2 data, we identified 38 PaO2 response and PaCO2 response patients (39.6%), 22 PaO2 response and PaCO2 non-response cases (22.9%), 13 PaO2 nonresponse and PaCO2 response subjects (13.5%), and 23 PaO2 nonresponse and PaCO2 nonresponse patients (24.0%). We found that both PaO2 and PaCO2 nonresponders had lower survival rates than other subgroups (Fig. 1C).
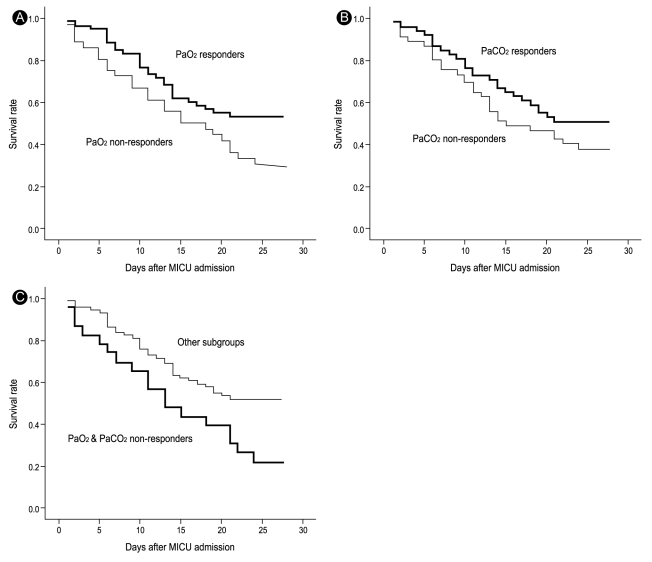
Kaplan-Meier estimates of survival at 28 days for severe acute respiratory distress syndrome patients under prolonged prone ventilation. (A) Comparison between PaO2 responders and nonresponders (log rank, 5.26; p = 0.022). (B) Comparison between PaCO2 responders and nonresponders (log rank, 1.49; p = 0.223). (C) Comparison between PaO2 and PaCO2 nonresponders and other subgroups (log rank, 6.14; p = 0.013). This figures shows the PaO2 responders, not the PaCO2 were associated with an improved 28-day outcome (A and B), and both PaO2 and PaCO2 nonresponders had lower survival rate than other subgroups (C).
MICU, medical intensive care unit.
However, no significant difference in clinical characteristics between PaO2 and PaCO2 responders and non-responders was observed (Table 3, data not shown for PaCO2 responders and nonresponders). Furthermore, no difference was found between survivors and non-survivors in ventilator parameters or gas exchange values collected just before or around 8 hours after first placement in the prone position (Table 4).
DISCUSSION
To our knowledge, this is the first study to investigate clinical characteristics and outcomes in severe ARDS patients under conditions of prolonged prone positioning in Korea. We found that all PaO2 responders showed oxygenation improvement soon after prone positioning, and those patients who maintained the oxygenation improvement at 8 to 12 hours after first prone positioning seemed to have better outcomes. Some of our study subjects underwent various adjuvant therapies for improved oxygenation, but no significant difference in the number of patients treated with adjuvant therapies or the duration of therapies was found between PaO2 responders and non-responders. Thus, our findings indicate that the oxygenation improvement was likely primarily due to prone positioning, not to the adjuvant therapies. We also observed a low rate of complications related to prolonged prone positioning, consistent with previously reported clinical trials [14,15,24]. Our data suggest that using the prone position for prolonged periods of time is both feasible and safe, and this reinforces the idea that a beneficial effect may be expected if prone oxygenation is applied early and continuously, as has been reported in some clinical studies [14,15].
However, these patients with prolonged prone positioning usually require continuous deep sedation using analgesics, sedative agents, and neuromuscular blocking agents, as seen in our study. This continuous deep sedation may be harmful to patients and may be associated with a prolongation of mechanical ventilation, which can increase a patient's likelihood of developing specific complications, including ventilator-associated pneumonia, barotraumas, airway complications (e.g., tracheal stenosis and ulceration), and unanticipated extubation, with resultant arterial oxygenation. It may also be risky because early recognition of brain injury through communication and neurologic examination often is severely limited [16,17,25]. Thus, our results may provide tips to physicians for predicting clinical parameter(s) associated with better outcomes in patients with prolonged prone ventilation.
Our data differ in some aspects from those in a previous report; Gattinoni et al. [18] reported that improved survival at 28 days was significantly associated with decreases in PaCO2, but not with PaO2 increases, during first session placement in the prone position. In our study, the PaCO2 response to the first prone positioning in ARDS patients with prolonged prone positioning was not associated with survival. Unlike the cases studied by Gattinoni et al. [18], all of our patients had severe ARDS, with much decreased alveolar ventilation and CO2 elimination. Accordingly, it is possible that our patients had a higher total minute ventilation/PaCO2 ratio (an approximate surrogate for the dead space tidal volume ratio) before prone positioning than did the patients in the study by Gattinoni et al. [18]. Also, many of our patients had pulmonary ARDS with increased stiffness of the thorax and insufficient alveolar ventilation.
Our study has several limitations. First, the study was retrospective in design, and we could not strictly control the various factor that affect the gas exchange response during prone ventilation (especially ventilator parameters). Second, we expected that specific subgroups formed by combining PaO2 and PaCO2 responders/non-responders to define four subgroups would have differed in outcome, and that those patients who had the most severe form of ARDS (SAPS II score > 49) would benefit from prone positioning; however, we were unable to draw such conclusions, possibly due to the small numbers of enrolled patients. Third, this study was carried out in only one hospital, and it may not represent the general situation.
In summary, we found that improved oxygenation at 8 to 12 hours (PaO2 response) in patients positioned prone for a long period (≥ 12 hours) was associated with an improved 28-day outcome. Thus, patients with severe ARDS who show initial PaO2 improvement (more than ≥ 20 mmHg) after prone ventilation seem to be appropriate candidates for prolonged prone ventilation.
Notes
No potential conflict of interest relevant to this article was reported.