Characteristics of Double-Chambered Right Ventricle in Adult Patients
Article information
Abstract
Background/Aims
This study evaluated the clinical features of double-chambered right ventricle (DCRV) in adults. Most cases of DCRV are diagnosed and treated during childhood. Consequently, very few reports include cases in which its clinical characteristics are evident in adults.
Methods
We reviewed the clinical data for 10 adult patients (age ≥ 18 years) with DCRV.
Results
Electrocardiogram showed right ventricular hypertrophy in 3 DCRV patients. All cases were associated with ventricular septal defect (VSD; 7 for perimembranous, 2 for muscular outlet, and 1 for the subarterial type). Surgical correction was done for 7 DCRV patients all of whom survived operations. Their follow-up echocardiogram showed the pressure gradient in their right ventricle was significantly decreased from 69.4 ± 17.2 mmHg preoperatively to 10.2 ± 5.0 mmHg postoperatively (p < 0.05). In the short-term follow-up, there was no significant increase in the pressure gradient in the right ventricle.
Conclusions
There are lots of cases of DCRV that are not diagnosed accurately in adults. In our experience, all DCRV cases had VSD and surgical correction of these cases showed excellent results. Therefore, accurate diagnosis of DCRV is necessary so that DCRV is not overlooked and operations are enabled within an appropriate time.
INTRODUCTION
Double-chambered right ventricle (DCRV) is a cardiac disease of the right ventricular outflow tract obstruction characterized by anomalous muscle bundles (AMB) that divide the right ventricle into two chambers, a high-pressure inflow chamber and a low-pressure outflow chamber. The origin of AMB has been debated [1-3]. Most cases of DCRV are diagnosed and treated during childhood. Furthermore, there is a tendency for the obstruction of the right ventricular outflow tract to progress if not treated adequately. However, very few reports include cases in which its clinical features, treatment and prognosis are in the adult age group [1,4-8]. Clinical characteristics of DCRV in adults are assessed in this current study.
METHODS
Study population
We searched the computerized database for all adult patients (age ≥ 18) diagnosed as DCRV with echocardiography or cardiac catheterization in our hospital between April 1999 and May 2006. Diagnostic information was collected by reviewing clinical records, electrocardiograms, echocardiograms and cardiac catheterization studies. Surgical findings were collected by review of operative notes. The follow-up information was obtained by reviewing clinical records and by telephone. Diagnosis of DCRV was based on the following criteria [6]: 1) a pressure gradient by echocardiogram or cardiac catheterization across the AMB within the right ventricle, 2) angiographic demonstration of anomalous obstruction within the right ventricle, 3) absence of infundibular hypoplasia, and 4) surgical confirmation of DCRV in the operating room. In order for a patient to be included in the study, the first 3 criteria or the fourth criterion had to be met.
Echocardiography and catheterization
Echocardiographic examination was performed before cardiac catheterization in all patients. Detailed analysis with a good echocardiographic window was possible in all patients in whom standard as well as modified views by 2D imaging were followed by Doppler assessment by color and continuous-wave Doppler, to note the site of flow acceleration and quantification of gradients across AMB. The peak pressure gradient was determined by Bernoulli's simplified equation. Right- and left-heart catheterization was carried out in all patients. Pressure withdrawal tracings were recorded by an end hole catheter from the pulmonary artery to the right ventricular outflow to the right ventricular inflow. Similarly left ventricular outflow gradients were recorded by the retrograde arterial approach. Right ventricular angiography was done in both anteroposterior and lateral projections. Cardiac catheterization and echocardiographic studies were also performed and interpreted by at least two special cardiologists for congenital heart disease (CHD).
Statistical analysis
Age and follow-up intervals are expressed as median and range. Group data are presented as the mean value ± standard deviation. The relationship among parameters was analyzed using the Spearman nonparametric test. For each of the analyses, a p value of < 0.05 was considered significant.
RESULTS
From the echocardiographic and cardiac catheterization data, ten patients were diagnosed as DCRV. Their median age was 29 years (range, 18 to 51). Three (30%) were female and 7 (70%) were male (Table 1). All but one complained of exertional dyspnea of grade II and more (patients 1, 3 to 10), palpitation (patient 9), and easy fatigability (patient 1, 4, 7); Patients with corresponding numbers can be found on Table 2. All patients had a history of a known "heart disease" since childhood but the exact diagnosis was unclear. Only one patient had a family history for CHD as his daughter was diagnosed with tetralogy of Fallot. On physical examination, all patients showed a loud, harsh, systolic ejection murmur of variable grade at the second and third left intercostal space. Chest X-ray showed mild cardiomegaly in five patients. The remaining patients had normal chest X-rays. Electrocardiogram (ECG) showed right ventricular hypertrophy in three DCRV patients, with extreme right axis deviation, tall R wave in V1, and negative deflection in aVR. Detailed echocardiography was performed in all patients. A definitive diagnosis was obtained in 9 patients by echocardiography, except for one who was falsely diagnosed with tetralogy of Fallot.
Echocardiographic and catheterization findings
The systolic gradient across the AMB by Doppler test ranged from 5.0 to 92.0 mmHg (mean, 38.9 ± 34.2). By cardiac catheterization (Fig. 1), it ranged from 7 to 101 mmHg (mean, 44.4 ± 40.4; Table 2). The preoperative ratio of right ventricular inflow to left ventricular pressure was 0.58 ± 0.34 (range, 0.27 to 1.10), which is correlated with the pressure gradient across the AMB (r = 0.89, p < 0.001). The pressure gradient across the AMB was not correlated with the pressure gradient across the ventricular septal defect (VSD), defect size of VSD and Qp / Qs (p > 0.05). In terms of the angiographic findings, in 9 patients the right ventriculogram showed an oblique and low obstruction (Fig. 2), and in 1 patient, the obstruction was a mixed type of high and low obstruction.
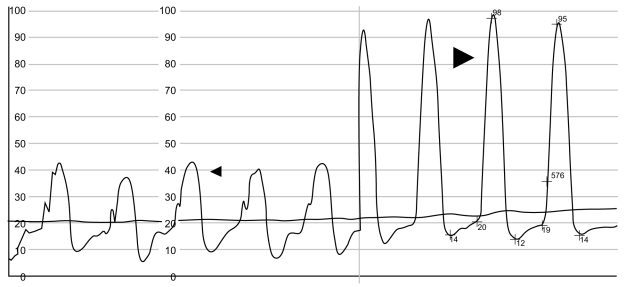
Right ventricular pressure tracing (patient 9). Thin arrow, infundibular (outflow chamber) systolic pressure of 42 mmHg. Thick arrow, apical (inflow chamber) systolic pressure of 98 mmHg.

Frontal view of the right ventriculogram (patient 7) at end-systole showing a low and oblique muscular obstruction. Anomalous muscle bundle divided the cavity into the two chambers. AMB, abnormal muscle bundle; MPA, main pulmonary artery; PV, pulmonary valve; RV, right ventricle; RVOT, right ventricle outflow tract.
Associated anomalies
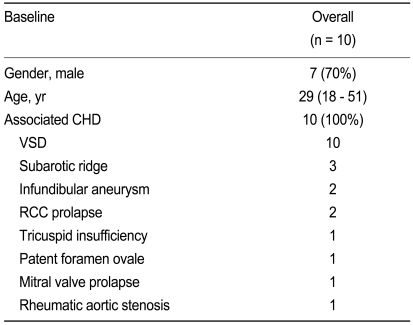
All cases were associated with ventricular septal defect (7 exhibited the perimembranous type [PM], 2 the muscular outlet [MO] type, and 1 had the subarterial [SA] type). Other associated anomalies included a subaortic ridge for 3, an infundibular aneurysm for 2, a prolapse of the right coronary cusps for 2, a tricuspid insufficiency for 1, a patent foramen ovale for 1, a mitral valve prolapse for 1 and a rheumatic aortic stenosis for 1 patient (Table 1).
Surgical operation
Surgical correction was carried out for 7 DCRV patients where the pressure gradient in the right ventricle was greater than 20 mmHg, or where they exhibited a significant VSD (Qp / Qs ≥ 2.0). Surgery consisted of a patch closure of the VSD and a resection of the AMB through the right atriotomy and pulmonary arteriotomy. In 3 patients with subaortic ridges, the resection for this was carried out through the aortic valve. Other associated procedures included aortic valve replacement with mechanical valves (21 mm, St. Jude Medical Inc., St. Paul, MN, USA) in 1 patient with severe aortic valve stenosis, and the other patient had an operation performed involving mitral and tricuspid annuloplasty (Tailor® annuloplasty ring, St. Jude Medical Inc.) with a primary closure of the patent foramen ovale.
Follow-up
All surgically corrected DCRV patients survived the operations. One patient showed an asymtomatic right bundle branch block on the electrocardiogram postoperatively, but normalized thereafter. The other patient showed symtomatic atrial fibrillation with a high-grade atrioventricular block postoperatively, and required an insertion of a single chambered permanent pacemaker. No significant clinical symptoms were documented at follow-up in this patient. In operated DCRV patients, postoperative echocardiograms showed that the pressure gradient across the AMB in the right ventricle was significantly decreased from 69.4 ± 17.2 mmHg preoperatively to 10.2 ± 5.0 mmHg (p < 0.05) (Fig. 3). Follow-up periods ranged from 5 to 10 months (median, 6 months) for patients who were not operated on. In 7 patients who underwent operations, the follow-ups ranged from 1 to 48 months (median, 2 months) post-operatively. There was no significant increase in the pressure gradient in the right ventricle in the 7 patients who were operated on, or on the 3 DCRV patients who did not undergo any operative procedures. No patients required further operations for obstruction of the right ventricular outflow tract.

Doppler echocardiographic assessments of changes in the pressure gradient across the abnormal muscle bundles in the right ventricle over time in 7 DCRV patients with the pressure gradient in the right ventricle greater than 20 mmHg (O), or with significant VSD, Qp / Qs ≥ 2.0 (X). DCRV, double-chambered right ventricle; VSD, ventricular septal defect; Op / Qs, pulmonary flow volume / systemic flow volume.
DISCUSSION
In the present study, DCRV is likely to be found more common in adult patients than previously reported, especially in patients with VSD. Excellent immediate postoperative results are apparent in selected patients.
Diagnosis
Most cases of DCRV are diagnosed and treated during childhood, and very few reports include cases in which the initial presentation for diagnosis and treatment occurs during adulthood [9-14]. It is difficult to diagnose DCRV with transthoracic echocardiography (TTE) [15]. In one study, of 32 patients, only five were diagnosed by means of TTE (15.6%) [16]. This reflected problems with delineating the morphology of the right ventricular outflow tract from that of the precordial planes owing to its proximity to the transducer, and other limitations encountered while studying adults, such as obesity and emphysema (Fig. 4). Therefore, it is critical to carry out echocardiography carefully in order to ascertain DCRV, especially when it is associated with a ventricular septal defect or with right ventricular hypertrophy on ECG. On Doppler examination, the pressure gradient across AMB with echocardiography was lower than that with cardiac catheterization techniques. This may be explained by the fact that quantitation of the gradients across AMB may sometimes be difficult because of the direction of flow acceleration, which is frequently at a significant angle to the direction of the ultrasound beam.

Transthoracic echocardiograph from a modified short axis view at aortic level (patient 9). Color flow Doppler imaging indicates the turbulent flow originating from the left ventricle through the ventricular septal defect to the abnormal thickened mass in the right ventricle, here accelerated in the mosaic pattern.
Classification and origin
A simple classification of the pathology in DCRV was proposed by Folger, who described two positions of the abnormal muscle bundle: high (or horizontal) and low (or oblique) [17]. It is well documented that right ventricular outflow tract obstruction caused by AMB may be an acquired phenomenon in some patients with ventricular septal defect and that the obstruction may progress with time. In our patients, AMB that were initially non-obstructive possibly became hypertrophic and obstructive with time due to the increased blood flow via VSD. As outlined by Wong et al. [3], the moderator band was found to be superiorly displaced in those infants with a VSD who eventually developed DCRV. No displacement of the moderator band was seen in age-matched patients with a VSD who did not develop DCRV. This suggests that the anatomic substrate for DCRV exists before the development of a pressure gradient. It has also been suggested that the increased blood flow and pressure within the right ventricular outflow tract may act as an initial stimulus for hypertrophy of the crista supraventricularis in patients with a VSD and with a notably increased pulmonary blood flow. We considered that left-to-right shunt occurring due to the ventricular septal defect could be the stimulus for the muscle bundles to develop obstruction with time. We have studied the characteristics of VSD effects on the DCRV. In the results, the pressure gradient across the AMB was not related to Qp / Qs, defect size and the pressure gradient across the VSD. The pressure gradient across the VSD has a tendency to be higher in DCRV patients, with the pressure gradient in the right ventricle less than 20 mmHg than in others more than 20 mmHg. It may be explained by the fact that the opening of the defect is directed to the right ventricular inflow tract in the case of PM typed VSD, and its blood flow across the VSD may stimulate the muscle bundles in RV to be hypertrophic and finally progress to the DCRV due to a significant pressure gradient.
Associated heart diseases
DCRV is exceptionally rare as an isolated anomaly [18]. Most commonly (in around 90% of cases), it is associated with a membranous type VSD [15]. The other coexisting lesions include subaortic stenosis, pulmonary valve stenosis, double outlet right ventricle, tetralogy of Fallot, anomalous pulmonary venous drainage, complete or corrected transposition of the great arteries, pulmonary atresia with intact ventricular septum, and Ebstein anomaly [19]. In our study, all cases were associated with VSD (7 for the PM type, 2 for the MO type, and 1 for the SA type). Other associated anomalies included subaortic ridges, aortic insufficiency, tricuspid insufficiency, infundibular aneurysms, patent foramen ovales, and mitral valve prolapses [20].
Surgical implications
The surgical repair of DCRV consists of resection for the AMB. In previous studies, in the absence of a significant coexisting defect, observation may be appropriate as long z 40 mmHg, and the obstruction is not progressive [5]. In our institute, a pressure gradient of greater than 20 mmHg in the right ventricle was the indicator for operational procedures. According to Oliver et al. [20], a mild right midventricular obstruction shows a fast rate of progression in adolescents and young adults. A right ventricular outflow tract obstruction in DCRV is likely to progress, and eventually lead to the patients becoming symptomatic. Although traditionally DCRV has been repaired transventricularly, this operative method may depress the right ventricular function and increase the risk of ventricular arrhythmias. Coates et al. [21] suggested that adequate exposure of the AMB could be obtained through the right atrium and tricuspid valve. However, because the RVOT may not be seen clearly, an undiagnosed DCRV may be missed. The preoperative diagnosis of DCRV is therefore crucial. Right ventricular outflow tract obstruction generally does not recur [8,22], although cases with recurrent obstruction have been described [23]. In our study, no significant increase of the RV pressure gradient was recorded at short-term follow-up.
DCRV has been reported as a rare disease in adults. However, cardiologists often do not consider and often overlook this anomaly. Consequently, there may be a number of cases that are not diagnosed properly or accurately. Careful evaluation of DCRV by echocardiography is necessary, especially in patients with VSD to ensure that DCRV is not overlooked. After proper diagnosis, operational procedures should be carried out within an appropriate time frame.
Notes
No potential conflict of interest relevant to this article was reported.