Variable Association between Components of the Metabolic Syndrome and Electrocardiographic Abnormalities in Korean Adults
Article information
Abstract
Background/Aims
Resting electrocardiogram (ECG) abnormalities have been strongly associated with cardiovascular disease mortality. Little is known, however, about the association between individual components of metabolic syndrome and ECG abnormalities, especially in Asian populations.
Methods
We examined clinical and laboratory data from 31,399 subjects (age 20 to 89 years) who underwent medical check-ups. ECG abnormalities were divided into minor and major abnormalities based on Novacode criteria. Ischemic ECG findings were separately identified and analyzed.
Results
The overall prevalence rates of ECG abnormalities were significantly higher in subjects with than in those without metabolic syndrome (p < 0.01). Ischemic ECG was strongly associated with metabolic syndrome in all age groups of both sexes, except for younger women. In multiple logistic regression analysis, metabolic syndrome was independently associated with ischemic ECG (odds ratio, 2.30 [2.04 to 2.62]; p < 0.01), after adjusting for sex, age, smoking, and family history of cardiovascular disease. Of the metabolic syndrome components, hyperglycemia in younger subjects and hypertension in elderly subjects were major factors for ischemic ECG changes, whereas hypertriglyceridemia was not an independent risk factor in any age group. The association between ischemic ECG findings and central obesity was weaker in women than in men.
Conclusions
Metabolic syndrome was strongly associated with ECG abnormalities, especially ischemic ECG findings, in Koreans. The association between each component of metabolic syndrome and ECG abnormalities varied according to age and sex.
INTRODUCTION
Metabolic syndrome refers to a constellation of ischemic heart disease risk factors, including central obesity, disorders of glucose and lipid metabolism, and hypertension, each of which has been shown individually to increase cardiovascular risk [1-6]. Furthermore, metabolic syndrome itself has been shown to increase cardiovascular and all-cause mortality in subjects without known heart disease [7-9]. Although the association between metabolic syndrome and cardiovascular disease (CVD) is well established, the magnitude of the association is highly variable according to the age, sex, and ethnicity of the study population.
Resting electrocardiogram (ECG) abnormalities have been strongly associated with subsequent all cause, coronary heart disease (CHD), and CVD mortality [10-13]. For example, the severity of abnormal ECG findings, defined as minor and major abnormalities by Novacode criteria, has been correlated with increasing risk for CHD events and mortality [13]. During recent decades, the rapid economic growth and lifestyle changes in many Asian countries, including South Korea, have led to increases in CVD and metabolic syndrome. Little is known, however, about the association between metabolic syndrome and ECG abnormalities, especially in Asian populations [14,15]. Moreover, variability in the association between each component of metabolic syndrome and ECG abnormalities has not been clarified. We therefore investigated the association between ECG abnormalities and metabolic syndrome in Korean adults. We also assessed the relationship between ECG abnormalities and individual metabolic syndrome components according to age and sex.
METHODS
Subjects
We examined clinical and laboratory data from 31,399 subjects (18,717 men and 12,682 women; median age, 48 years [range, 20 to 89]) who visited the Health Promotion Center at Asan Medical Center for medical check-ups in 2006. Each participant was administered a standard questionnaire that assessed alcohol intake, smoking habits, usual patterns of physical activity, medical history, family history of diabetes and ischemic heart disease, and regular medication. This study was approved by the Institutional Review Board of the Asan Medical Center.
Measurements
Height and weight were measured while subjects were wearing light clothing without shoes. Waist circumference was measured midway between the costal margin and the iliac crest at the end of a normal expiration. Blood pressure was measured with a mercury sphygmomanometer on the right arm with subjects in the sitting position after a 5 minutes rest. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Blood samples were obtained in the morning after an overnight fast. Plasma glucose was measured by the hexokinase method using an autoanalyzer (Hitachi, Tokyo, Japan). Standard liver function testing, total cholesterol, high-density lipoprotein (HDL) cholesterol, low-density lipoprotein (LDL) cholesterol, and triglycerides were also measured using an autoanalyzer (Hitachi).
Definition of metabolic syndrome
Metabolic syndrome was diagnosed using the 2005 revised National Cholesterol Education Program - Adult Treatment Panel III (NCEP-ATP III) criteria [16] with the Asian specific cutoff point for abdominal obesity [17]. According to these criteria, subjects with metabolic syndrome are those with any combination of three or more of the following risk determinants: fasting plasma glucose ≥ 5.6 mmol/L or on antidiabetic treatment, blood pressure ≥ 130 / 85 mmHg or on antihypertensive treatment, plasma triglycerides ≥ 1.7 mmol/L, plasma HDL cholesterol < 1.03 mmol/L in men and < 1.29 mmol/L in women, and waist circumference > 90 cm in men and > 80 cm in women.
ECG analysis
Standard 12-lead ECGs were recorded with each subject in the supine position using strictly standardized procedures. Special attention was paid to locating the chest electrodes at precise positions [18]. All ECGs were inspected visually to detect technical errors, missing leads, and inadequate quality. ECGs were coded by a cardiology specialist using the Novacode system [19].
ECG abnormalities were divided into minor and major abnormalities based on Novacode criteria [19]. Minor ECG abnormalities included any of the following: 1) first- and second-degree atrioventricular block (Novacode 2.1 and 2.2.1); 2) borderline prolonged ventricular excitation (Novacode 3.4.1 and 3.4.2); 3) prolonged ventricular repolarization (Novacode 4.1.1 and 4.1.2); 4) isolated minor Q and ST-T abnormalities (Novacode 5.7 and 5.8); 5) left ventricular hypertrophy without ST-T abnormalities (Novacode 6.1.0); 6) left atrial enlargement (Novacode 7.1); 7) frequent atrial or ventricular premature beats (Minnesota code 8.1); and 8) fascicular blocks (Novacode 10.1 and 10.2). Major ECG abnormalities included any of the following: 1) atrial fibrillation or atrial flutter (Novacode 1.5); 2) high-degree atrioventricular dissociation (Novacode 2.3.1 and 2.3.2); 3) left bundle-branch block (Novacode 3.1.0 and 3.1.1); 4) right bundle-branch block (Novacode 3.2.0); 5) indeterminate conduction delay (Novacode 3.3.0 and 3.3.1); 6) Q-wave myocardial infarction (MI) (Novacode 5.1, 5.2, 5.3, and 5.4); 7) isolated ischemic abnormalities (Novacode 5.5 and 5.6); 8) left ventricular hypertrophy with ST-T abnormalities (Novacode 6.1.1); and 9) miscellaneous arrhythmias (e.g., supraventricular tachycardia, ventricular preexcitation, ventricular tachycardia; Novacode 1.4, 1.7, 1.8, 1.9, and 2.4).
'Ischemic ECG' findings (signs suggesting MI or ischemia) were defined as the presence of Q/Qs patterns, significant or borderline ST segment depression, deep or moderate T wave inversion, or evidence of complete left bundle-branch block [20]. 'Non-ischemic ECG abnormalities' were defined as all other minor or major ECG abnormalities.
Statistics
Data are expressed as means ± SD. Variables not normally distributed, such as blood concentrations of glucose and triglycerides, were log-transformed before analysis. A chisquare (χ2) test was used to compare prevalence rates of ECG abnormalities between groups with and without the metabolic syndrome. Logistic regression analyses were used to find independent factors for ischemic ECG and to obtain odds ratio after adjusting for several clinical and biochemical variables. Statistical analyses were performed using the SPSS version 14.0 (SPSS Inc., Chicago, IL, USA). A p value of less than 0.05 was considered statistically significant.
RESULTS
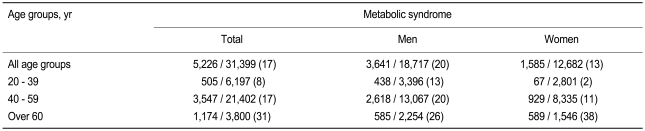
The clinical and laboratory characteristics of the study subjects are listed in Table 1. Of the 31,399 subjects, 5,226 (17%) had metabolic syndrome (20% for men and 13% for women), defined by modified ATP III criteria with Asia-Pacific guidelines for waist circumference. Using the original ATP III waist circumference criteria (102 cm in men and 88 cm in women), the prevalence of metabolic syndrome was 12% (13% for men and 11% for women). Table 2 lists the prevalence of metabolic syndrome according to sex and age. The prevalence of metabolic syndrome in subjects > 60 years old was markedly higher (26% in men and 38% in women) than in younger age (< 60 years) groups, especially in women.
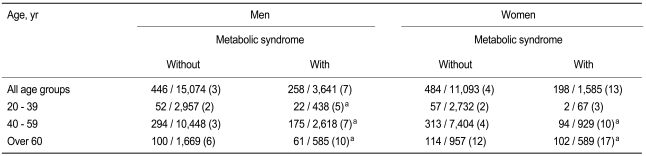
According to the Novacode criteria, 4% (6% of men, 3% of women) of the subjects had major ECG abnormalities, and 16% (19% of men, 12% of women) had minor abnormalities. Both major and minor ECG abnormalities were more prevalent in subjects with metabolic syndrome than in those without (p < 0.01 each by χ2 test) (Table 3). Ischemic ECG abnormalities were identified in 4% of the subjects (4% of men, 5% of women), and non-ischemic ECG abnormalities in 17% (21% of men, 11% of women). The prevalence rates of ischemic (9% vs. 4%, p < 0.01 by χ2 test) and non-ischemic ECG (20% vs. 16%, p < 0.01 by χ2 test) abnormalities were significantly higher in subjects with metabolic syndrome. Ischemic ECGs showed a strong association with metabolic syndrome in all age groups of both sexes, except for women < 40 years old (Table 4).
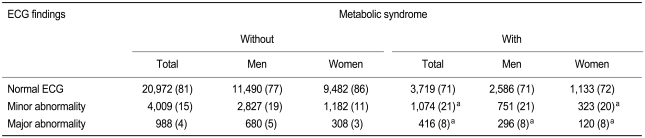
Prevalence of major and minor ECG abnormalities by Novacode criteria in subjects with or without metabolic syndrome
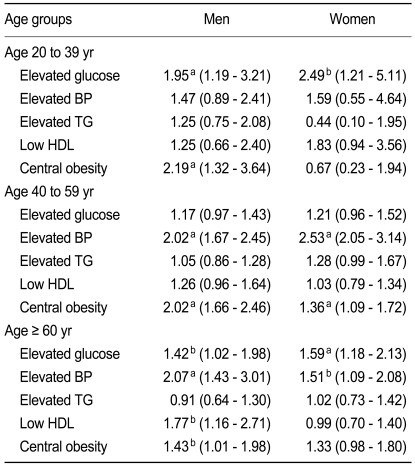
In a multiple logistic regression analysis, metabolic syndrome was independently associated with ischemic ECG (odds ratio [OR], 2.30; 95% confidence interval, 2.04 to 2.62, p < 0.01) after adjusting for sex, age, smoking, and family history of CHD (Table 5). We also used multiple logistic regression analyses to calculate the OR for each component of the metabolic syndrome in different sex and age groups (Table 6). Among the various components of metabolic syndrome, hyperglycemia and central obesity in men < 40 years old and hyperglycemia in women < 40 years old were main risk determinants for ischemic ECG, whereas hypertension and low HDL cholesterol in men > 60 years old and hypertension in women > 60 years old were additional risk factors for ischemic ECG. Elevated triglyceride level was not an independent risk factor for ischemic ECG in any subgroup. Low HDL cholesterol was also not a significant risk factor in women. In addition, the association of ischemic ECG findings with central obesity was much weaker in women than in men, especially in younger age groups.
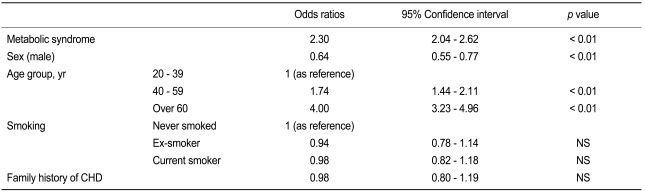
Adjusted odds ratios for factors associated with ischemic ECG, as determined by multiple logistic regression analysis
DISCUSSION
In this cross-sectional study of a large number of Korean subjects, we showed that metabolic syndrome was strongly associated with ECG abnormalities, especially ischemic ECG findings, in a Korean population. This association was independent of other important risk factors for coronary artery disease, including subject age, smoking history, and family history of CHD. These results are consistent with previous findings in other populations, i.e., that metabolic syndrome is associated with an increased risk of cardiovascular morbidity and mortality [8,21-23].
A unique finding of this study is that the association between ischemic ECG and each component of metabolic syndrome varied significantly according to age and sex. We found that hyperglycemia was the main risk determinant for ischemic ECG findings in younger (< 40 years old) subjects, whereas hypertension was important in older groups. In contrast, hypertriglyceridemia was not an independent risk factor in any age group, a finding consistent with a recent report showing that elevated triglyceride levels are not associated with 10-year CVD risk in a middle-aged Chinese population [24]. We also found that the association between ischemic ECG changes and central obesity was weaker in women than in men, especially in younger women. It was suggested that the waist circumference criterion for diagnosing metabolic syndrome may be too strict for Asian women [25]. The optimal cutoff value of waist circumference for Asian women should therefore be determined by long-term prospective population-based studies.
Using the Asia-Pacific criteria for waist circumference, the overall prevalence of metabolic syndrome in our study subjects was 17%, which is lower than that in the US (24% in men, 23% in women) [26], but similar to findings in other Korean [27] and Chinese [25] populations. In addition, the prevalence of ischemic ECG abnormalities in our study population (4% for men, 5% for women) was lower than in Iranian [14] and Belgian [20] populations (8 to 10% for men, 10 to 15% for women). The latter difference may be due to differences in ethnicity, age distribution, and / or lifestyle of the study subjects. Consistent with the concept that metabolic syndrome is a marker of cardiovascular risk, the prevalence of metabolic syndrome was correlated with that of ischemic ECG abnormalities.
Interestingly, we found that the prevalence of metabolic syndrome was much higher in elderly (> 60 years) women (38%) than in elderly men (26%), a finding consistent with previous results in Korean [27,28] and Chinese [25,29] populations, but different from findings in a US population [26] that reported similar prevalences in elderly men and women. Moreover, the prevalence of ischemic ECG abnormalities was higher in elderly women (14%) than in elderly men (7%). These results are consistent with previous reports showing a higher prevalence of ischemic ECG [20] and cardiovascular disease [30] in elderly women than in elderly men. Taken together, these data on the high prevalence of metabolic syndrome in elderly women reflect the higher risk of cardiovascular disease in this group.
Our study had several limitations. First, the study was based only on resting ECG findings, not on clinically symptomatic or angiographically documented coronary artery disease. Baseline ECG abnormalities, however, were found to be significantly associated with subsequent coronary artery disease [11,12,31]. In addition, the low cost, wide availability, and ease of interpretation makes ECG a useful tool in large population studies. The second limitation of our study was that our study subjects were not a random sample of the general population; however, the subjects we assessed may be representative of a modernized urban population increasingly encountered in developed or developing countries, and the large sample size may reduce any potential selection bias. Third, our analysis was cross-sectional, not longitudinal, suggesting a need for additional follow-up to confirm the causal relationship between metabolic syndrome and CVD. It would also be useful to investigate the role of metabolic syndrome in the prediction of cardiovascular event by a follow-up ECG study. Despite these limitations, our results indicate that identification of metabolic syndrome is an important and useful clinical tool for screening subjects with high cardiovascular risks, which can lead to increased efforts to maintain a healthy lifestyle. Thorough evaluation for latent CVD and careful follow up would also be warranted for subjects with metabolic syndrome and ECG abnormalities.
In conclusion, these results show that metabolic syndrome is strongly associated with ECG abnormalities, especially ischemic ECG findings, in a Korean population; however, the relationship between each component of metabolic syndrome with ischemic ECG changes varied according to age and sex. Further studies are required to redefine the criteria for hypertriglyceridemia and central obesity in Asian populations.
Notes
No potential conflict of interest relevant to this article was reported.