Modified Number of Extranodal Involved Sites as a Prognosticator in R-CHOP-Treated Patients with Disseminated Diffuse Large B-Cell Lymphoma
Article information
Abstract
Background/Aims
Rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone chemotherapy (R-CHOP) has improved survival in patients with diffuse large B-cell lymphoma (DLBCL) and weakened the prognostic power of the international prognostic index (IPI). We evaluated the efficacy of the IPI and revised IPI (R-IPI) in patients with DLBCL who were treated with R-CHOP, focusing on extranodal site number (ENS) because extranodal involvement occurs frequently in Koreans.
Methods
A total of 126 R-CHOP-treated patients with stage III/IV DLBCL were analyzed. We performed a retrospective analysis of the clinicopathologic factors and verified the predictive power of the standard IPI and R-IPI. Various numbers of extranodal sites were analyzed for further stratification, and we set the extranodal site-modified IPI (E-IPI) as the IPI when the number of extranodal sites was stratified as < 3 vs. ≥ 3.
Results
A univariate analysis showed that ENS was associated with complete response (CR, p = 0.04), event-free survival (EFS, p = 0.01), and overall survival (OS, p < 0.001) when the ENS cut-off was set at ≥ 3. A multivariate analysis revealed that an ENS ≥ 3 remained associated with EFS (p < 0.01; hazard ratio [HR], 2.60; 95% confidence interval [CI], 1.29 to 5.26) and OS (p < 0.01; HR, 3.52; 95% CI, 1.68 to 7.35). The IPI was effective for determining prognosis in terms of OS (p = 0.04) but not EFS (p = 0.17). The R-IPI was effective in terms of both variables (p = 0.02 and 0.04, respectively), as was the E-IPI (p = 0.01 and 0.001, respectively).
Conclusions
An ENS < 3 vs. ≥ 3, rather than the original < 2 vs. ≥ 2, was the most significant prognostic factor for EFS and OS. All three indices were predictive, but only the E-IPI identified the high-risk group of R-CHOP-treated Korean patients with disseminated DLBCL.
INTRODUCTION
After cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) chemotherapy was introduced in the 1970s, it became the standard treatment regimen for patients with diffuse large B-cell lymphoma (DLBCL). More intensive regimens were designed subsequently, but did not lead to additional survival benefits [1]. However, when rituximab was developed and combined with CHOP chemotherapy (R-CHOP), the survival of patients with DLBCL was dramatically improved [2-4].
DLBCL is characterized by a variety of clinical features, and the prognosis of this disease varies depending on these features. Because high-risk patients may benefit from alternative or intensive strategies, it is important to classify patients with DLBCL by their prognosis. For this purpose, many clinical factors and biomarkers have been evaluated and suggested as prognostic factors [5]. This led to the international prognostic index (IPI), which is based on five clinical factors; age > 60 years, stage III/IV disease, > 1 extranodal site, European Cooperative Oncology Group (ECOG) performance status ≥ 2, and elevated serum lactate dehydrogenase (LDH) levels [6]. Until recently, the IPI was the standard indicator that was generally used in practice and clinical trials to predict patient outcomes [6].
Korean patients with DLBCL have extranodal involvement much more frequently than patients in other countries do (50 to 60% vs. about 30%) [7-11]. This more prevalent extranodal involvement suggests that, for Koreans, the cut-off number of extranodal sites indicating a poor prognosis should be higher than the cut-off applied in the IPI (≥ 2). However, systematic studies examining this issue have not been performed.
Furthermore, several reports have shown that R-CHOP chemotherapy overcomes factors that were found previously to indicate a poor prognosis, which means that the standard IPI has become less effective for predicting the outcomes of R-CHOP-treated patients [2,12-15]. The widespread use of R-CHOP indicates that the hitherto widely accepted DLBCL prognostic factors should be reevaluated. Indeed, a number of recent studies have sought to identify new factors that allow a more effective determination of the prognosis of DLCLB patients [12,14,16]. One of these, a study in British Columbia, showed that the revised IPI (R-IPI) is a better indicator of prognosis than the standard IPI for R-CHOP-treated patients with DLBCL [15]. In the R-IPI, patients are classified with a very good, good, or poor prognosis depending on whether they have 0, 1 to 2, or 3 to 5 IPI factors, respectively [15]. The superior prognostic effectiveness of R-IPI was recently confirmed by a subsequent study [14].
Here, we performed a retrospective analysis to define prognostic factors for R-CHOP-treated patients with DLBCL in Korea. In particular, we sought to identify the appropriate extranodal site cut-off number that maximizes the prognostic significance of this factor for the Korean population. We also assessed the predictive power of the standard IPI, the R-IPI, and a modified form of the IPI (E-IPI) in which the extranodal site number cut-off was set at ≥ 3.
METHODS
Patients
We searched the Asan Medical Center (Seoul, Korea) lymphoma database and identified newly diagnosed patients with stage III/IV CD20+ DLBCL, who were treated with R-CHOP as first-line chemotherapy. In total, 126 patients who were diagnosed from January 2002 to May 2008 were included in this retrospective analysis. All pathology records were reviewed by a hematopathologist (JH) and classified according to the WHO classification system. The clinical and demographic data were collected by reviewing the patients' medical records.
A staging evaluation was performed according to the Ann Arbor staging system. This involved performing a physical examination, determining complete blood counts and differential white blood cell counts, assessing the biochemical profile (including LDH levels), and performing a bilateral bone marrow aspiration and biopsy, a computed tomography (CT) scan of the neck, thorax, abdomen and pelvis, and whole-body positron emission tomography.
IPI factors were identified from the clinical data according to a previous report [6]. The patients were then assigned to standard IPI groups, which consisted of low-risk (1 IPI factor), intermediate-risk (2 to 3 IPI factors), and high-risk (4 to 5 IPI factors) groups. In a separate analysis, the patients were divided into R-IPI groups, which consisted of good- (1 to 2 IPI factors) and poor-prognosis (3 to 5 IPI factors) groups [15]. As this study involved only stage III and IV patients, all patients included in this analysis had at least one IPI factor. Consequently, none of the patients fell into the very good-prognosis group (0 IPI factors).
All patients were treated with R-CHOP and were intended to receive six to eight cycles of chemotherapy. Rituximab (375 mg/m2) was administered at 3-week intervals with the standard dose of CHOP.
Statistical analysis
The primary outcomes of this study were complete response (CR), event-free survival (EFS), and overall survival (OS). CR was determined according to the conventional response criteria [17]. EFS was estimated from the date of initial chemotherapy to the date of disease progression, relapse, or death. OS was defined from the date of initial chemotherapy to the date of death. Lost to follow-up was the date of last follow-up. Chi-square and Fisher's exact tests were used to identify the CR predictive factors. EFS and OS were estimated according to the Kaplan-Meier method, and survival curves were compared by the log-rank test. In the multivariate analysis, multiple logistic regression analysis was conducted for CR, and the Cox proportional hazard model was performed to assess the independent prognostic factors for EFS and OS. The SPSS version 14.0 (SPSS Inc., Chicago, IL, USA) was used for all statistical analyses, and all tests were two-sided. Statistical significance was defined as p < 0.05.
RESULTS
Patient characteristics
The clinicopathological characteristics of the 126 patients with stage III/IV DLBCL are listed in Table 1. The median age at diagnosis was 57 years (range, 16 to 81).
Treatment
In total, 61% of the patients finished six to eight cycles of R-CHOP. The remaining patients failed to complete the intended chemotherapy due to progression or relapse of the disease or because the toxicity of the regimen was intolerable. Thirteen percent of the patients were treated with radiotherapy. Radiotherapy was usually used for treating residual masses that were retained after the full R-CHOP course had been completed or for palliation of the symptoms caused by the tumor masses. Patients whose disease relapsed or progressed after first-line chemotherapy received salvage chemotherapy with a variety of regimens.
Prognostic factors used in the univariate and multivariate analyses
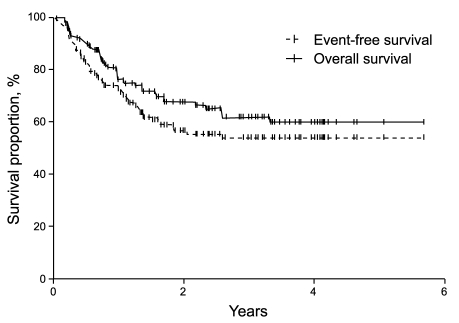
With a median follow-up time of 22.4 months (range, 0.5 to 69.3), the CR rate was 69%, the 2-year EFS was 56%, and the 2-year OS was 67.4% in all patients (Fig. 1). In the univariate analysis, when the extranodal site number cut-off was set at ≥ 3, the presence of three or more extranodal sites was significantly associated with a poor prognosis for CR (p = 0.04), EFS (p = 0.01), and OS (p < 0.001). Age > 60 years was also significantly associated with a poor prognosis for EFS (p = 0.03) (Table 2, Fig. 2). When the extranodal site number cut-off was set at ≥ 2, this variable was no longer significantly associated with CR (p = 0.08), EFS (p = 0.47), or OS (p = 0.35). In the multivariate analysis, using the IPI factors but with the extranodal site cut-off set at ≥ 3, the extranodal site number remained significantly associated with EFS (p < 0.01; hazard ratio [HR], 2.60; 95% confidence interval [CI], 1.29 to 5.26) and OS (p < 0.01; HR, 3.52; 95% CI, 1.68 to 7.35). Age > 60 years also remained significantly associated with a poor EFS (p = 0.02; HR, 1.95; 95% CI, 1.12 to 3.41) (Table 3).
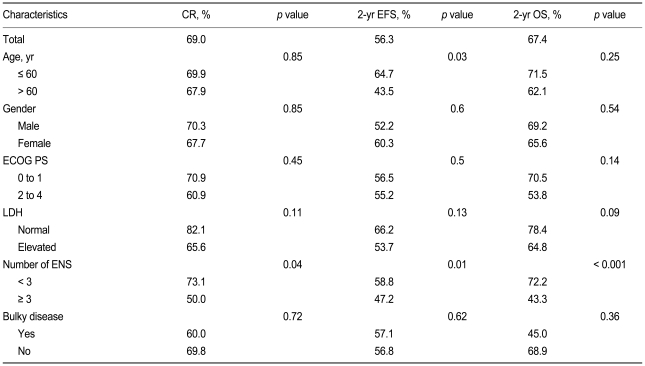
Univariate analysis of complete response, 2-year event-free survival, and 2-year overall survival in relation to patient characteristics
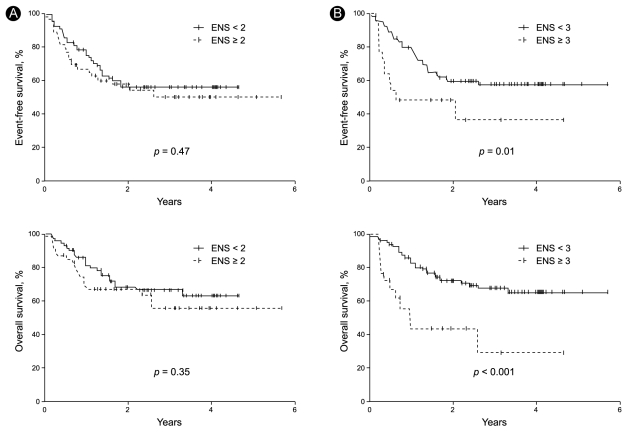
Event-free survival and overall survival curves when the patients were stratified according to whether they had (A) < 2 vs. ≥ 2 extranodal sites (ENS) or (B) < 3 vs. ≥ 3 ENS.
Outcomes of patients classified according to the standard IPI and the R-IPI
To determine the efficacy of the standard IPI, the patients were stratified into low- (1 IPI factor), intermediate- (2 to 3 factors), and high-risk (4 to 5 factors) groups. The three risk groups had a 2-year EFS of 68%, 55%, and 56% (p = 0.17), respectively, and a 2-year OS of 85%, 68%, and 58% (p = 0.04), respectively (Table 4, Fig. 3). Thus, the standard IPI was effective for determining the prognosis of R-CHOP patients with DLBCL for OS but not for EFS.
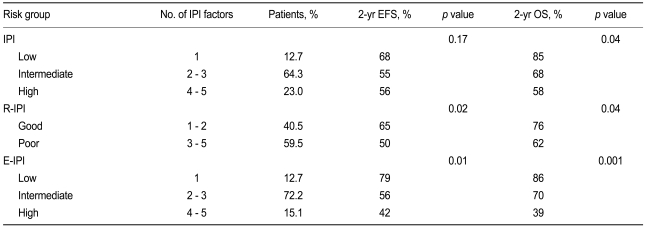
Event-free survival and overall survival values of R-CHOP-treated patients after classifying them according to the standard IPI, the R-IPI, and the E-IPI
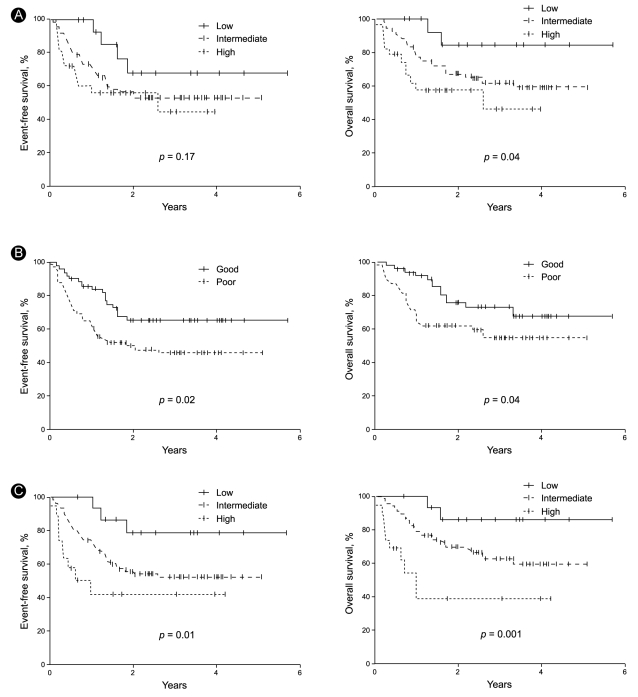
Event-free survival and overall survival curves of patients classified according to the standard international prognostic index (IPI, A), the revised IPI (R-IPI, B), and the extranodal site-modified IPI (E-IPI, C).
To determine the efficacy of the R-IPI, the patients were regrouped into good- (1 to 2 IPI factors) and poor-prognosis (3 to 5 factors) groups. It should be noted that the R-IPI normally includes three risk groups, but only two risk groups were employed in this study because all of our patients were stage III and IV patients and could not belong to a risk group characterized by the absence of IPI factors. The good- and poor-prognosis groups had a 2-year EFS of 65% and 50% (p = 0.02), respectively, and a 2-year OS of 76% and 62% (p = 0.04), respectively (Table 4, Fig. 3). Thus, the R-IPI was of good prognostic relevance for both EFS and OS for R-CHOP-treated patients with DLBCL.
Outcomes according to the E-IPI, in which the extranodal site number cut-off was set at ≥ 3
As indicated above, the number of extranodal sites was more effective as a prognostic factor when it was stratified as < 3 and ≥ 3 than when it was stratified as < 2 and ≥ 2. To assess this further, we modified the IPI by adjusting the cut-off of the number of extranodal sites so that ≥ 3 extranodal sites indicated a poor prognosis. We refer to this modified IPI as the extranodal site-modified IPI (E-IPI). Based on the E-IPI, we redivided the patients into low- (1 IPI factor), intermediate- (2 to 3 IPI factors), and high-risk (4 to 5 IPI factors) groups (Table 4). The E-IPI was of good prognostic value for both EFS and OS, as the 2-year EFS values of the low-, intermediate-, and high-risk groups were 79%, 56%, and 42%, respectively (p = 0.01), and the 2-year OS values for these groups were 86%, 70%, and 39% (p = 0.001), respectively (Table 4, Fig. 3). Significantly, unlike the standard IPI and the R-IPI, the E-IPI identified a group of patients whose survival rates were below 50%.
DISCUSSION
Much effort has been expended to define a prognostic index for DLBCL because pretreatment risk assessment helps to choose the initial treatment and to predict outcomes. The IPI is the standard indicator that, until recently, was generally used in practice and clinical trials to predict the outcomes of patients with DLBCL. Because the treatment of choice for patients with DLBCL has changed from CHOP to R-CHOP, the overall outcomes of these patients has improved remarkably [2,4]. This change in standard treatment has led to attempts to reevaluate the clinical and biological prognostic factors that were relevant in the CHOP era [12-14,16]. Recently, two reports showed that the predictive power of IPI was reduced in patients with R-CHOP-treated DLBCL, and new prognostic models based on a modification of the IPI were suggested [14,15]. It is also possible that the relevance of the prognostic markers varies depending on the geographic area and patient ethnicity due to varying baseline clinical characteristics of the patients. Compared to patients in other countries, Koreans have higher rates of extranodal involvement in DLBCL, which is one of the five clinical IPI factors [7-11]. Such geographic and ethnic variation suggests that Korean patients may differ from patients of other ethnicities in terms of the degree of extranodal involvement that is of prognostic significance [18].
Here, we sought to identify the cut-off extranodal site number that was prognostically relevant for R-CHOP-treated Korean patients with disseminated DLBCL. The standard IPI states that ≥ 2 extranodal site indicates a poor prognosis. Indeed, a recent report showed that R-CHOP-treated patients with DLBCL in Singapore who had ≥ 2 extranodal sites had a lower CR and 2-year survival than patients with 0 or 1 extranodal site, although this variable did not remain significant after a multivariate analysis [14]. The same study also showed that male gender and advanced stage were significantly associated with a poor prognosis. In contrast, in this study on Korean patients, we found that lymphoma involvement of ≥ 3 extranodal sites was significantly associated with a poor prognosis for EFS and OS upon both univariate and multivariate analysis, whereas setting the cut-off rate at ≥ 2 was of no prognostic relevance. The disparity between our study and the Singapore study may relate to the different prevalences of extranodal site involvement in Singapore and Korea. In addition, although we also evaluated the prognostic relevance of other IPI factors for our Korean patients, only age > 60 years had power to predict a poor EFS in this cohort. These results support the notion that the prognostic factors and their power to predict disease outcome may vary for patients with DLBCL who differ in geography and ethnicity. Thus, to predict the prognosis of a specific cohort more precisely, the individual prognostic factors may need to be modified and optimized to improve their predictive power.
For our patients, who had advanced stage DLBCL and who were treated with R-CHOP, the standard IPI remained effective as a prognostic model for OS but could not predict EFS. Similarly, a previous report by a British Columbia study group showed that the predictive power of the standard IPI was diminished when used with R-CHOP-treated patients with DLBCL [15]. As a result, those authors developed the R-IPI, in which the five IPI factors are redistributed, and the patients are classified into very good-, good-, and poor-prognosis groups [15]. In the present study, we found that the R-IPI had power to predict both the EFS and OS of our cohort, although it should be noted that our patients could only be classified into good- and poor-prognosis groups because we excluded patients with localized disease so as to achieve treatment homogeneity.
Significantly, we found that the E-IPI provided more relevant information about the prognosis of Korean patients with DLBCL than did the standard IPI or the R-IPI. Because the overall prognosis of DLBCL has improved greatly since the introduction of R-CHOP, it has become difficult to identify patients who are at higher risk for a poor prognosis [2,4,15]. Indeed, we found that the standard IPI and R-IPI failed to identify a high-risk group of R-CHOP-treated DLBCL patients that had a survival rate < 50%. This agrees with observations in other studies, although the present study cannot be compared directly to previous reports because of the relatively shorter follow-up period and the inclusion of advanced stage patients only [14,15]. In contrast, the E-IPI identified a patient group in our cohort that had a 2-year survival of only 39%. Although these observations need to be validated by additional studies performed in other regions and with different ethnic groups, the E-IPI appears to be promising for determining the prognosis of Korean DLBCL patients in the R-CHOP era.
However, our observations may be of limited applicability with regard to patients with DLBCL in general. This is in part because our sample size was relatively small due to the exclusion of patients with localized disease so that we could analyze patients who were treated uniformly. Moreover, this study was also limited because biologic markers were not evaluated sufficiently, although there are few biologic markers for R-CHOP-treated patients [5,12,16]. Further investigation is needed to determine the applicability of our observations for different regions and ethnicities, especially in countries where extranodal site involvement of DLBCL is more common.
In conclusion, setting the number of extranodal sites at < 3 vs. ≥ 3, rather than the original < 2 vs. ≥ 2, was the most significant prognostic factor for EFS and OS of R-CHOP-treated Korean patients with stage III/IV DLBCL. Although the standard IPI, R-IPI, and E-IPI were all predictive of survival, the E-IPI was clinically more useful for identifying the high-risk group in this cohort. These observations must be studied further to determine their general applicability.
Acknowledgements
This study was presented at 14th Congress of the European Hematology Association (as a poster presentation).
Notes
No potential conflict of interest relevant to this article was reported.