Comparison of Clinical Outcomes by Different Renal Replacement Therapy in Patients with End-Stage Renal Disease Secondary to Lupus Nephritis
Article information
Abstract
Background/Aims
Many studies have compared patients with systemic lupus erythematosus (SLE) on renal replacement therapy (RRT) with non-lupus patients. However, few data are available on the long-term outcome of patients with end-stage renal disease (ESRD) secondary to SLE who are managed by different types of RRTs.
Methods
We conducted a retrospective multicenter study on 59 patients with ESRD who underwent maintenance RRT between 1990 and 2007 for SLE. Of these patients, 28 underwent hemodialysis (HD), 14 underwent peritoneal dialysis (PD), and 17 patients received kidney transplantation (KT). We analyzed the clinical outcomes in these patients to determine the best treatment modality.
Results
The mean follow-up period was 5 ± 3 years in the HD group, 5 ± 3 years in the PD group, and 10 ± 5 years in the KT group (p = 0.005). Disease flare-up was more common in the HD group than in the KT group (p = 0.012). Infection was more common in the PD and HD groups than in the KT group (HD vs. KT, p = 0.027; PD vs. KT, p = 0.033). Cardiovascular complications were more common in the HD group than in the other groups (p = 0.049). Orthopedic complications were more common in the PD group than in the other groups (p = 0.028). Bleeding was more common in the HD group than in the other groups (p = 0.026). Patient survival was greater in the KT group than in the HD group (p = 0.029). Technique survival was lower in the PD group than in the HD group (p = 0.019).
Conclusions
Among patients with ESRD secondary to SLE, KT had better patient survival and lower complication rates than HD and lower complication rates than PD. The prognosis between the HD and PD groups was similar. We conclude that if KT is not a viable treatment option, any alternative treatment should take into account the patient's general condition and preference.
INTRODUCTION
Lupus nephritis develops in 60% of patients with systemic lupus erythematosus (SLE), and 20% of patients with lupus nephritis develop end-stage renal disease (ESRD) within 10 years of disease onset [1]. Although renal involvement in SLE is frequent and important, patients with ESRD secondary to SLE are relatively uncommon. As such, few studies have focused on patients having SLE treated with renal replacement therapy (RRT), and choosing the appropriate RRT is an important consideration to maximize survival and quality of life in these patients.
Previous studies have reported similar cumulative survival rates between patients with SLE and non-SLE patients who received renal RRT [2-4]. These studies provide a limited comparison across a small number of RRT modalities and focus on changes in disease activity. Limited evidence exists on the long-term clinical course of these patients treated with different RRT modalities, including kidney transplantation (KT). Additionally, since most reviews on patients with SLE treated using RRT rely on meta-analysis outcomes, controversies exist regarding the clinical courses and survival rates in patients treated with different RRT modalities [1].
To address this controversy, we conducted a retrospective multicenter study evaluating the clinical courses and survival of patients with SLE managed by three different RRT modalities. Results based on these data were used to determine the best treatment modality among three RRTs in patients with ESRD secondary to SLE.
METHODS
Patient enrollment and characteristics
We examined the medical records of patients diagnosed with SLE, according to criteria established by the American College of Rheumatology [5], who underwent RRT in Korea from 1990 to 2007 at four tertiary medical centers: Seoul St. Mary's Hospital, St. Mary's Hospital, Uijeongbu St. Mary's Hospital, and St. Vincent's Hospital. Patients who received RRT for less than 3 months were excluded from this study. Of the 59 patients enrolled in the study, 28 received hemodialysis (HD), 14 had peritoneal dialysis (PD), and 17 underwent KT. The following data were documented from patient records: gender, age, onset of disease, duration of RRT, predominant RRT prior to KT (defined as the one used for > 50% of the total duration of ESRD prior to KT [6]), disease flare-up (defined as an increase of ≥ 1.0 on a physician's global assessment as compared to the previous visit [7]), infection, malignancy, cardiovascular accident, orthopedic disease, bleeding, newly developed and/or aggravated diabetes mellitus (DM) or hypertension (HTN), and survival. Except DM and HTN, only hospital admission records were included in this study. In case of aggravated DM and HTN, the doses and number of medications before RRT were compared with that prescribed at their last visit after RRT.
Statistical analysis
Data were analyzed using SPSS version 15.0 (SPSS Inc., Chicago, IL, USA). Comparisons of events among the HD, PD, and KT groups were performed using one-way analysis of variance (ANOVA) and an independent t test. In the present study, we introduced episode/patient-year to adjust for different follow-up periods and compared this ratio using a t test. Patient, technique, and graft survival estimates were calculated using the Kaplan-Meier analysis. Survival estimates between groups were compared with the log-rank test; p < 0.05 was considered to be statistically significant.
RESULTS
Baseline characteristics
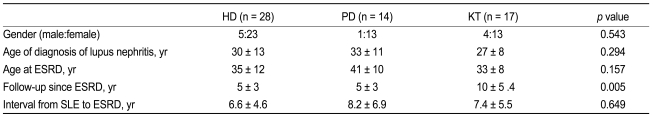
Demographic characteristics of patients following ESRD onset are shown in Table 1. The population studied was predominantly female (83%). Age at SLE diagnosis was 30 ± 13 years in the HD group, 33 ± 11 years in the PD group, and 27 ± 8 years in the KT group. Age at ESRD onset in the HD, PD, and KT groups were 35 ± 12, 41 ± 10, and 33 ± 8 years, respectively. The intervals from SLE diagnosis to ESRD were 6.6 ± 4.6 years in the HD group, 8.2 ± 6.9 years in the PD group, and 7.4 ± 5.5 years in the KT group. The mean follow-up periods of the HD, PD, and KT groups were 5 ± 3, 5 ± 3, and 10 ± 5 years, respectively. The HD, PD, and KT groups all showed similar mean age of SLE onset and interval from SLE diagnosis to ESRD. The mean follow-up period of the KT group was longer than that of the HD and PD groups (p = 0.005). Prior to undergoing KT, 10 patients predominantly underwent HD and 6 patients predominantly received PD. One patient did not have dialysis prior to KT. The durations of dialysis prior to KT were 30 ± 32 months for HD and 36 ± 39 months for PD. No significant difference was observed in the duration of RRT prior to KT.
Clinical outcomes analyses
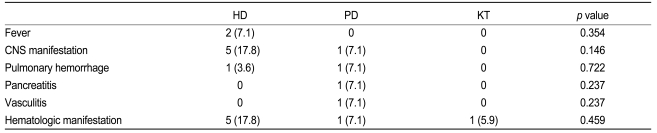
Table 2 summarizes the patterns of disease flare-up in the study patients. SLE flare-up occurred in 9 patients who underwent HD (29%, 0.52 episode/patient-year), 4 patients who received PD (32%, 0.22 episode/patient-year), and 1 patient who had a KT (6%, 0.01 episode/patient-year). Disease flare-up was more common in the HD group than in the KT group (p = 0.012) and the episode/patient-year remained the same. No significant difference in disease flare-up was observed among the other groups. Patients on HD and PD developed complications including fever, central nervous system (CNS) manifestation of SLE, pulmonary hemorrhage, hematologic manifestation, vasculitis, and pancreatitis. Only 1 patient who had a KT developed hematologic manifestations of SLE.
Table 3 summarizes the infection episodes in the study patients. Infection occurred in 13 patients in the HD group (46%, 0.64 episode/patient-year), 11 patients in the PD group (79%, 0.36 episode/patient-year), and 10 patients in the KT group (59%, 0.15 episode/patient-year). When comparing the three groups, infection by episode/patient-year was more common in the HD and PD groups than in the KT group (HD vs. KT, p = 0.027; PD vs. KT, p = 0.033). These events included pneumonia, tuberculosis, viral infection, sepsis, skin and soft tissue infection, and fungal infection. Pneumonia was the most common infection in the HD and PD groups (14% and 14%, respectively), and viral infection was the most common infection in the KT group (35%). Severe infection such as tuberculosis or sepsis was more common in the HD group. Cardiovascular complications occurred in 8 patients in the HD group (29%, 0.18 episode/patient-year), 2 in the PD group (14%, 0.09 episode/patient-year), and none in the KT group. Comparing the three groups, cardiovascular complications were more common in the HD group than in the PD and KT groups (p = 0.049), although no significant difference was observed by episode/patient-year. When comparing the HD and KT groups, cardiovascular complications were more common in the HD group (p = 0.004), although no difference was observed by episode/patient-year. Five patients in the HD group developed an arteriovenous fistula problem. Dilated cardiomyopathy, uncontrolled hypertension, and heart failure developed in one patient in the HD group. Orthostatic hypotension and heart failure developed in one patient who received PD.
Additional complications were noted in the study population, including malignancies, orthopedic disease, bleeding, aggravated DM, and HT. Malignancy occurred in 3 patients in the HD group (11%, 0.03 episode/patient-year) who were diagnosed with breast, stomach, or cervical cancer. None of the patients in the PD group developed a malignancy and 2 in the KT group (12%, 0.01 episode/patient-year) developed stomach cancer or thymoma. Orthopedic complications occurred in 1 patient in the HD group (4%, 0.002 episode/patient-year), 3 in the PD group (21%, 0.07 episode/patient-year), and 2 patients in the KT group (12%, 0.01 episode/patient-year). Orthopedic complications by episode/patient-year were more common in the PD group than in other groups (p = 0.028). One patient in the HD group developed rhabdomyolysis of unknown cause, 2 patients in the PD group developed avascular necrosis of the femur head (AVN), 1 patient in the PD group developed compression fracture of the spine, and 2 patients in the KT group developed AVN.
Bleeding occurred in 8 patients in the HD group (29%, 0.22 episode/patient-year), none in the PD group, and 1 patient in the KT group (6%, 0.004 episode/patient-year). Bleeding was more common in the HD group than in other groups (p = 0.026), although the difference was not significant (p = 0.053) by episode/patient-year. When comparing bleeding between the HD group and the KT and PD groups, it was more common in the HD group than in the others (HD vs. KT, p = 0.033; HD vs. PD, p = 0.004). Similar results were obtained by episode/patient-year. In the HD group, 7 patients developed gastrointestinal bleeding, 2 developed bleeding of the vascular access, and 1 patient developed vaginal bleeding. One patient in the KT group developed postoperative hematoma in the perinephric space.
Aggravated DM occurred in 2 patients (7%, 0.04 episode/patient-year) in the HD group, no patients in the PD group, and 2 patients in the KT group (18%, 0.02 episode/patient-year). Aggravated HTN occurred in 9 patients on HD (32%, 0.25 episode/patient-year), 6 PD patients on PD (43%, 0.18 episode/patient-year), and 8 patients who received a KT (47%, 0.44 episode/patient-year). No significant difference was observed in aggravated HTN and/or DM.
Survival analysis
Cumulative survival rates at 5 and 10 years were 100% and 90% in the KT group, 93% and 81% in the PD group, and 79% and 55% in the HD group, respectively (Fig. 1). When comparing two groups separately, survival was higher in the KT group than in the HD group (p = 0.029), but no significant difference was observed between the HD and PD groups (p = 0.334) in survival rates.
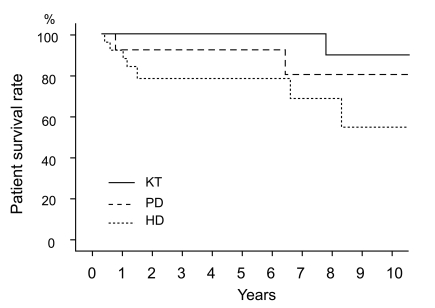
Patient survival according to the treatment modality. Survival was higher in the KT group than in the HD group (p = 0.029, tested by log rank test), but no significant difference was observed between the HD group and the PD group (p = 0.334). KT, kidney transplantation; PD, peritoneal dialysis; HD, hemodialysis.
Twelve deaths were recorded and Table 4 shows the causes of death by RRT modality. Disease flare-up, infection, cardiovascular disease, malignancy, and bleeding were the causes of death in patients with SLE on RRT. Regarding the PD group, 2 patients died of disease flareup (CNS lupus, infection following pancytopenia), 1 died due to infection, and another succumbed to cardiovascular disease. In the HD group, 2 patients died of cardiovascular disease, 2 died due to malignancy, 1 died of disease flare-up (pulmonary hemorrhage), 1 died due to infection, and 1 patient died due to bleeding. Except for 1 patient who succumbed to recurrent thymoma, no other patient died in the KT group.
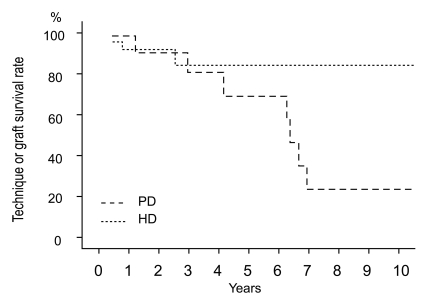
Technique or graft survival rates at 5 and 10 years were 89% and 89% in the KT group, 70% and 23% in the PD group, and 86% and 86% in the HD group, respectively (Fig. 2). Technique survival rate was lower in the PD group than in the HD group (p = 0.019). The causes of technique failure in the PD group were peritonitis in 5 patients, perihepatic abscess in 1 patient, and recurrent pancreatitis in 1 patient. The causes of technique failure in the HD group were vascular access infection in 1 patient and bleeding of vascular access in another patient. Rejection occurred in 1 patient who underwent KT.
DISCUSSION
The age at ESRD onset is much younger in patients with SLE than in the general RRT population [8,9]. Thus, choosing the appropriate RRT modality is important to maximize survival and the quality of life in patients with ESRD secondary to SLE. We conducted a retrospective multicenter study evaluating the clinical courses and survival of patients with SLE managed by three different RRT modalities to determine the best treatment option among three RRTs in patients with SLE and ESRD. The results showed that KT had a higher survival and lower complication rates than HD and lower complication rates than PD. We found that, in most complications such as disease flare-up, infection, orthopedic complication, bleeding, and survival rate, KT is superior to HD and PD. Note, however, that the mean follow-up period of the KT group was longer than that of the other groups. When comparing HD with PD, PD was better than HD across most study outcomes except in orthopedic complications. However, the technique survival rate of PD was lower than that of HD and we believe that patients undergoing PD received an early modality change following failure of the procedure.
Disease activity in patients with SLE that progressed to ESRD has been studied extensively, and SLE disease activity was found to decline following RRT commencement, except in PD in most studies [1-3,10,11]. This study showed that SLE flare-up in patients on RRT was less common in the KT group. Although issues regarding immunosuppressant use are not mentioned in the current study, most patients in the KT group had been using calcineurin inhibitors and steroids, whereas most patients in the other groups only received steroids. Thus, we think that disease flare-up control was associated with proper immunosuppressant use, such as calcineurin inhibitors [12,13], and bioincompatibility by the dialysate or dialyzer. Our data also determined that KT was superior to HD and PD in infection control and severity. Because of the immunosuppressive regimens administered to prevent allograft rejection after KT, and the immunosuppressive actions of ESRD prior to transplantation, several investigators mistakenly assumed that infections were more common in patients who underwent KT [14]. The infection risk of patients in the KT group was actually the smallest among RRTs, especially severe infections including tuberculosis and sepsis, although less severe infections including viral infections were more common. The reason for this low infection risk after KT remains unclear. Contrary to KT, patients with SLE on dialysis are known to be at an increased risk of infection compared to non-lupus patients on dialysis [15].
No significant differences were observed in cardiovascular complications or malignancies between RRT modalities. Hemodynamic instability, aneurysm of vascular access, and thrombosis in the HD group may have been related to frequent cardiovascular complications, consistent with previous studies [3]. Patients with ESRD secondary to SLE have little comorbidity due to short disease duration, rapid disease progression, and relatively young age. Therefore, the pattern of cardiovascular disease is different from that in non-lupus patients. Critical cardiovascular diseases such as coronary heart disease are more common in non-lupus patients than in patients with SLE. As survival improves, the pattern of cardiovascular disease will be more critical in patients with SLE. We hypothesize that the finding no differences in malignancies was due to the rarity of events. Most studies have not focused on malignancy because it is not the main cause of morbidity and mortality in patients with SLE and ESRD. Similar to cardiovascular diseases, as survival improves, malignancy will become an important issue in these patients and longer-term follow-up will be required.
Differences between treatment modalities were observed among orthopedic complications and bleeding risk. AVN was the main orthopedic complication in patients with SLE and ESRD and AVN was more common in the PD group than in the others. When previous studies involving patients with SLE and ESRD were reviewed, uremia, rejection, and steroid dosage were related to AVN [16-18]. However, controversies exist regarding the dosage of steroids used. With the exception of a few studies that focused on KT, few data exist on the orthopedic complications in patients with SLE and ESRD. Thus, further investigations are needed to establish AVN incidence and risk factors. Bleeding was more common in the HD group. Previous studies have investigated thrombotic complications related to the presence of antiphospholipid antibodies [3,19] because renal allograft thrombosis and vascular access were the main causes of morbidity in patients having SLE with antiphospholipid antibodies. In this study, none of the patients had antiphospholipid antibodies, but bleeding risks in the HD group were higher, which may have been related to anticoagulation factors such as heparin for HD.
SLE with renal involvement is a known risk factor of DM and HTN [20,21]. However, only small amounts of data are available on the differences among the three treatment modalities and the incidence of DM and HTN. Comparisons using simple changes in numbers or doses of medication are not the best way to make an objective determination and the current study was influenced by differences in the time of follow-up. Aggravated DM or HTN was not significantly different between groups when adjusted for follow-up period. However, more meaningful data will be obtained with a longer follow-up of the HD or PD group.
When previous studies on survival were reviewed, the cumulative survival rates following development of ESRD were 83-92% at 5 years in patients receiving HD, 53-80% in patients treated with PD, and 85-97% in patients receiving KT [3,4,19,22]. Compared to other studies, survival in the HD group in this study was poorer, whereas survival of patients in the PD group was better, while survival of patients in the KT group was similar to that of the PD group. We hypothesize that differences in patient survival between the HD and PD groups were due to two factors. First, HD is the preferred treatment for patients in poor general condition, which is related to poor survival rates. Second, long-term follow up of patients in the PD group in this study indicate good disease control and thus better survival rates. This is in contrast to reports that in the short term, disease control in patients receiving PD is poor but not significantly different from that in patients undergoing HD when compared over longer periods of time [3]. When compared to other studies, the present study showed longer time on dialysis prior to KT (HD, 30.3 ± 32.3 months; PD, 36.6 ± 39.6 months). All transplanted kidneys came from living donors and advanced immunosuppressants such as tacrolimus were used [4,6,22,23]. Note that controversies regarding the relationship between dialysis time and KT prognosis exist and since disease control prior to KT is critical to survival, we hypothesized that long dialysis time pre-KT implied good disease control. Comparing the study conducted by Ward [4] with that of Moroni et al. [22], longer dialysis time was associated with better survival (dialysis time, 21 months vs. 42 months; 5 year survival, 83% vs. 97%, respectively). However, further investigations are needed to substantiate this association. Considering good patient survival rates and a low rate of perioperative and postoperative risks associated with KT (except for one perinephric hematoma recovered by conservative care), we recommend that KT should not be delayed.
In conclusion, the present study was aimed at assessing the long-term clinical outcomes of patients with ESRD secondary to SLE using adequate numbers of patients. This study focused on subjective or objective clinical outcomes in a multicenter retrospective design and did not rely simply on disease activity. Our results demonstrate that KT has better survival and lower complication rates than HD and lower complication rates than PD. The prognosis was similar when comparing HD with PD since PD was better than HD across most study outcomes, although the technique survival rate of PD was lower than HD. Thus, if KT is not a viable option, we believe that an alternative treatment option should take into consideration the patient's general condition and preference. Limitations, however, exist to interpreting the data. The first was our inability to control variables such as age, immunosuppressant use, and disease duration in a retrospective format. The second was that when comparing survival rates, we disregarded the duration of RRT prior to KT. Finally, due to the rarity of ESRD secondary to lupus nephritis, we could not recruit a sufficient number of patients to give the study adequate statistical power. These limitations can be overcome with additional prospective studies that take into account these variables and recruit sufficient numbers of patients.
Notes
No potential conflict of interest relevant to this article was reported.