Detection of Clopidogrel Hyporesponsiveness Using a Point-of-Care Assay and the Impact of Additional Cilostazol Administration after Coronary Stent Implantation in Diabetic Patients
Article information
Abstract
Background/Aims
Impaired responsiveness to clopidogrel is common in patients with type 2 diabetes mellitus (DM). The aim of this study was to evaluate the clinical application of a point-of-care assay to detect impaired responsiveness to clopidogrel after coronary stent implantation in patients with type 2 DM.
Methods
We measured P2Y12 reaction units (PRU) with the VerifyNow point-of-care assay in 544 consecutive patients undergoing dual or triple (i.e., dual plus cilostazol) anti-platelet therapy after coronary stent implantation. High platelet reactivity (HPR) was defined as a PRU value ≥ 240.
Results
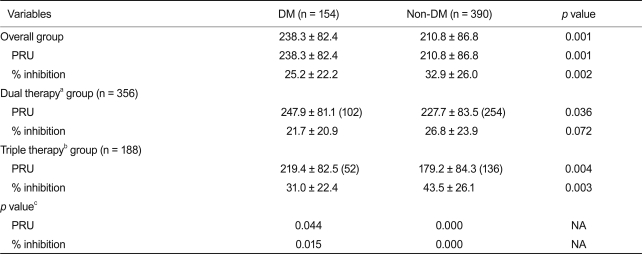
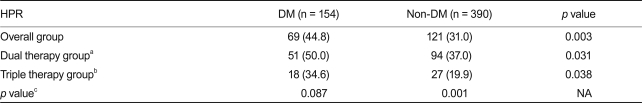
The mean PRU values were 233.5 ± 83.2 and 190.3 ± 85.5 in patients undergoing dual or triple anti-platelet therapy, respectively (p < 0.001). Patients with DM manifested higher post treatment PRU values (238.3 ± 82.4 vs. 210.8 ± 86.8, p = 0.001) and a higher frequency of HPR (44.8% vs. 31.0%, p = 0.003) as compared to patients without DM. We also found that higher PRU values and a higher frequency of HPR were present in patients with DM who were undergoing both triple and dual anti-platelet therapy. However, the higher post-treatment PRU values observed in patients with DM decreased with triple anti-platelet therapy (219.4 ± 82.5 vs. 247.9 ± 81.1, p = 0.044).
Conclusions
A point-of-care assay can detect elevated platelet reactivity and impaired responsiveness to clopidogrel in patients with type 2 DM. The addition of cilostazol to dual anti-platelet therapy may decrease post-treatment PRU values in patients with type 2 DM.
INTRODUCTION
Patients with type 2 diabetes mellitus (DM) have a higher risk of cardiovascular events and death than those without DM [1-3]. In addition, type 2 DM is a risk factor for stent thrombosis after drug-eluting stent implantation [4]. Elevated platelet reactivity, among other mechanisms, contributes to the increased risk of atherothrombotic complications in patients with type 2 DM [5,6]. Elevated platelet reactivity is more frequent in patients with type 2 DM than in those without DM, even when treated with dual anti-platelet therapy, such as aspirin and clopidogrel [7,8]. Cilostazol, a phosphodiesterase III inhibitor, has additional inhibitory effects on adenosine diphosphate (ADP) P2Y12 receptor-induced platelet aggregation when used with dual anti-platelet therapy [9,10].
Platelet reactivity traditionally has been measured using light transmittance aggregometry; however, problems with the clinical application of this method limit its routine use. The VerifyNow P2Y12 point-of-care assay (Accumetrics, San Diego, CA, USA) enables more simple and rapid measurements of platelet reactivity. Recent studies have shown that elevated platelet reactivity after clopidogrel therapy, as measured by a point-of-care assay, was associated with a higher risk of adverse events after percutaneous coronary intervention (PCI) [11,12].
The aim of this study was to evaluate the clinical application of a point-of-care assay to detect impaired clopidogrel responsiveness in patients with type 2 DM undergoing dual or triple (dual plus cilostazol) anti-platelet therapy after a PCI with stent implantation. In addition, the effects of cilostazol on platelet reactivity in patients with type 2 DM were evaluated.
METHODS
Study population
This study was conducted on 544 consecutive patients who underwent coronary stent implantation. Patients were enrolled in three hospitals (Inje University Busan Paik Hospital, Yeungnam University Hospital, and Keimyung University Dongsan Hospital) under a PCI registry. Patients who had received a loading dose of 300 mg or 600 mg clopidogrel and 200 mg aspirin at least 12 hours prior to the PCI or were on maintenance doses of 75 mg clopidogrel and 100 mg aspirin per day for more than 5 days were included in the study. Patients considered as having DM were those taking oral hypoglycemic agents or those who needed of insulin for adequate glucose control at the time of the PCI. Clinical data, including the results of the point-of-care assays, were collected prospectively. Exclusion criteria were as follows: 1) an ST segment elevation myocardial infarction while undergoing primary PCI; 2) a platelet count < 100 × 103/µL; 3) a serum creatinine > 2 mg/dL, and 4) patients who received periprocedural glycoprotein IIb/IIIa inhibitors. The local institutional review committees approved this study and all patients provided informed consent.
Medications
Coronary lesions were treated using standard PCI techniques. All patients received a daily maintenance dose of 75 mg clopidogrel and 100 mg aspirin. In addition, cilostazol, 200 mg daily, was prescribed in some patients. The addition of cilostazol was left to the discretion of the operators. Stent type selection and the use of glycoprotein IIb/IIIa antagonists were also left to the discretion of the operators. All patients received heparin during the procedure to maintain an activated clotting time ≥ 250 seconds and were prescribed lifelong aspirin.
Point-of-care assay
Venous blood was taken from each patient at least 2 days after the PCI and blood was drawn into a Greiner Bio-One 3.2% citrate Vacuette tube® (Greiner Bio-One, Monroe, NC, USA) and run within 60 minutes. The VerifyNow P2Y12 assay is a turbidmetry-based optical detection device that measures platelet responsiveness to clopidogrel and other P2Y12 antagonists. This system contains fibrinogen-coated polystyrene beads and measures changes in light transmission and thus the rate of aggregation in whole blood. This device has two whole-blood assay channels. One channel contains 20 µmol/L of ADP as an agonist. This channel also contains prostaglandin E1 as a suppressor of intracellular free calcium, which reduces the nonspecific contribution of ADP binding to P2Y1 receptors. Another separate channel contains an iso-thrombin receptor activating protein as an agonist. All results are expressed as P2Y12 reaction units (PRU). High platelet reactivity (HPR), as assessed by the point-of-care assay, was defined as a PRU value ≥ 240 according to the results of recent studies [11,12].
Statistical analysis
Comparisons were performed using a Student's t test or Mann-Whitney test and a chi-square or Fisher's exact test. A Kolmogorov-Smirnov test was used to test for normality. A multivariate logistic regression analysis was performed to identify independent predictors of HPR. All statistical tests were two-tailed and a p value < 0.05 was considered statistically significant. All calculations were performed using SPSS version 13 (SPSS Inc., Chicago, IL, USA).
RESULTS
From July 2007 to March 2009, we enrolled 544 consecutive patients in the current analysis. This study population included 154 diabetic patients. The baseline characteristics of all subjects are listed in Table 1. The diabetic group included 18 patients (11.7%) who needed insulin for adequate blood glucose control. Patients with DM had a higher frequency of hypertension (p = 0.01) and a lower level of creatinine clearance (p = 0.01) as calculated using the methods of Cockcroft and Gault, compared to those without DM. Patients with DM also had a longer stent length per lesion (p = 0.01) and a greater stent number per patient (p = 0.02) compared to those without DM. However, there were no significant differences in other coronary risk factors or medications, such as lipid lowering agents and anti-platelet agents.
Post-treatment PRU values were normally distributed (one-sample Kolmogorov-Smirnov test, p = 0.23). The mean post-treatment PRU values were 233.5 ± 83.2 and 190.3 ± 85.5 in patients undergoing dual or triple anti-platelet therapy, respectively (p < 0.001). Patients with DM had a higher post-treatment PRU compared to those without DM (238. 3 ± 82.4 vs. 210.8 ± 86.8, p = 0.001) (Fig. 1). Patients with DM also had a higher frequency of HPR compared to those without DM (44.8% vs. 31.0%, p = 0.003) (Fig. 2). The percentage of patients included in the 4th quartile of post-treatment PRU values was higher in patients with DM as compared to those without DM (32.5% vs. 23.3%, p = 0.03). Table 2 shows that higher post-treatment PRU values were observed in patients with DM undergoing both triple and dual anti-platelet therapy. The magnitude of the difference in PRU values between patients with and without DM was larger in patients undergoing triple anti-platelet therapy compared to those undergoing dual anti-platelet therapy. In patients with DM, the post-treatment PRU value was significantly lower in patients undergoing triple anti-platelet therapy as compared to those undergoing dual anti-platelet therapy (219.4 ± 82.5 vs. 247.9 ± 81.1, p = 0.044). Table 3 shows that patients with DM had a higher frequency of HPR regardless of the type of anti-platelet therapy. The frequency of HPR in patients with DM tended to be lower in patients undergoing triple anti-platelet therapy as compared to those undergoing dual anti-platelet therapy (34.6% vs. 50.0%, p = 0.087). DM was found to be a significant predictor of HPR in a multivariable analysis (odds ratio [OR], 1.76; 95% confidence interval [CI], 1.19 to 2.62; p = 0.004). In contrast, patients undergoing triple anti-platelet therapy (OR, 0.47; 95% CI, 0.31 to 0.70; p = 0.001) or who were smokers (OR, 0.63; 95% CI, 0.42 to 0.94; p = 0.022) were less likely to have HPR.

Post-treatment P2Y12 reaction units (PRU) values according to diabetic status and anti-platelet therapy. The difference between diabetes mellitus (DM) and non-DM patients was significant (p = 0.001). The lines inside the boxes denote the medians. The boxes mark the interval between the 25th and 75th percentiles.
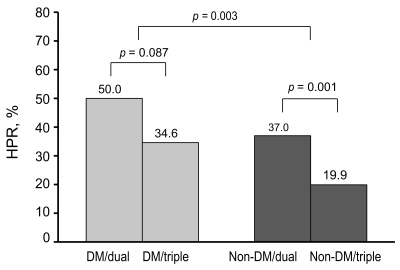
Frequency of high platelet reactivity (HPR) according to diabetic status and anti-platelet therapy. The difference between diabetes mellitus (DM) and non-DM patients was significant (p = 0.002).
There was no significant difference in post-treatment PRU values (256.2 ± 114.2 vs. 234.6 ± 76.7, p = 0.299) or the frequency of HPR (55.6% vs. 42.4%, p = 0.455) based on the treatment modality, i.e., either insulin or oral hypoglycemic agents. In addition, there was no significant difference in post-treatment PRU values (241.3 ± 82.9 vs. 231.8 ± 81.9, p = 0.504) or the frequency of HPR (46.7% vs. 40.8%, p = 0.602) based on the status of diabetic control, which was based on a hemoglobin level of A1c 7.0.
DISCUSSION
The current study included a large number of consecutive patients recruited from real-world practice. Within this study population, a point-of-care assay could detect elevated post-treatment platelet reactivity and impaired responsiveness to clopidogrel in patients with type 2 DM compared to those without DM. The current study shows 1) higher post-treatment PRU values and 2) a higher frequency of HPR in patients with DM compared to patients without DM. Furthermore, higher post-treatment PRU values and a higher frequency of HPR were observed in patients with DM undergoing triple anti-platelet and dual anti-platelet therapy. However, the higher post-treatment PRU values observed in diabetic patients were significantly decreased with triple anti-platelet therapy as compared to dual anti-platelet therapy.
Several studies have reported that impaired platelet responsiveness to clopidogrel is more frequent in patients with type 2 DM [7,8,13]. Insulin normally inhibits platelet aggregation via inhibition of the P2Y12 pathway. However, patients with type 2 DM show reduced responsiveness to insulin, leading to up-regulation of the P2Y12 pathway and elevated platelet reactivity, which results in reduced responsiveness to anti-platelet therapy [6,8,14]. Reduced responsiveness to clopidogrel has been associated with adverse outcomes after PCI, including stent thrombosis [15-18]. Furthermore, the wide inter-individual variability in the inhibitory effects of clopidogrel is well-established [19,20]. Therefore, evaluation of individual clopidogrel responsiveness has become common in patients undergoing PCI, especially in patients with higher risks, such as those with DM.
Several methods have been used to evaluate clopidogrel responsiveness to identify patients at a higher risk of adverse events [21]. Light transmittance aggregometry following several agonist stimuli has been considered the gold standard method for platelet function analysis in previous studies; however, the routine use of this method in clinical practice is difficult because of the need for well-trained personnel and blood sample centrifugation [22]. Vasodilator-stimulated phosphoprotein phosphorylation as measured by flow cytometry also has limitations in clinical practice, although this method has a higher specificity as compared with that of aggregometry [22].
Recently, efforts have focused on formulating appropriate and cost-effective platelet function tests to measure platelet reactivity and clopidogrel hyporesponsiveness so that anti-platelet therapy can be individualized. A point-of-care assay specifically measures the inhibitory effects of clopidogrel on P2Y12 platelet receptor activation. Point-of-care assay results have been shown to be closely correlated with ADP-induced light transmittance aggregometry [23-25]. Several studies have shown that post-treatment PRU values ≥ 235 or 240, as measured by a point-of-care assay, are associated with an increased risk of post-PCI adverse events, including cardiac death, myocardial infarction, and stent thrombosis [11,12,26]. Our findings show a higher frequency of HPR in patients with DM as compared to those without DM. These results are consistent with a study reported by Price et al. [26], in which the frequency of high platelet reactivity (PRU ≥ 235) was significantly higher than lower platelet reactivity in patients with DM. Similar findings were also reported in a study by Marcucci et al. [12], where the frequency of residual platelet reactivity (PRU ≥ 240) was significantly higher in patients with DM.
The appropriate management of impaired clopidogrel responsiveness in drug-eluting stent treatment remains to be determined. A high clopidogrel maintenance dose can increase platelet inhibition in patients treated with PCI [27], including those with DM [28]. Another approach to consider might be the addition of a phosphodiesterase III inhibitor such as cilostazol to dual anti-platelet therapy. One study showed that the addition of cilostazol to dual anti-platelet therapy resulted in higher ADP-induced platelet inhibition as compared to dual anti-platelet therapy alone [9]. Another study showed that additional cilostazol reduced the rate of high post-treatment platelet reactivity and intensified platelet inhibition as compared to a high maintenance dose of clopidogrel (150 mg/day) [29]. Previous studies used light transmittance aggregometry to demonstrate higher platelet inhibition with triple anti-platelet therapy as compared to dual anti-platelet therapy in the general population [9,29]. In contrast, our findings showed that adjunctive cilostazol has additional anti-platelet effects in patients with type 2 DM as measured with a point-of-care assay.
There was no significant difference in post-treatment PRU values or the frequency of HRP depending on the type of treatment modality or the status of diabetic control (based on a hemoglobin level of A1c 7.0) in the patients with DM. Because a smaller number of patients were treated with insulin and a greater number of patients had relatively good diabetic control in the current study, further studies are warranted to examine patients treated with insulin as compared to those with uncontrolled DM.
The limitations of the current study include the following. First, the current study was based on an analysis of registry data; therefore, there were some discrepancies in the period of use of the anti-platelet agents from onset of anti-platelet therapy to the platelet function test among individual patients. However, a relatively large number of study subjects were assessed and there was no statistical difference in the period of use of anti-platelet agents. Second, because the current study was not a randomized trial, there may be a selection bias in the analysis of triple anti-platelet therapy effects in patients with DM. Third, the current study focused on the results of a point-of-care assay; therefore, the clinical effects, according differences in the degree of platelet inhibition, were not assessed in association with the results of the point-of-care assay. Additional prospective, randomized studies including clinical data are planned at our institution. Finally, the mean post-treatment PRU value in patients undergoing dual anti-platelet therapy was somewhat higher than that reported in two previously published studies [12,26]. One study identified a higher prevalence of cytochrome P450 2C19 genetic polymorphisms in an Oriental population as compared with westerners, which could be a possible explanation for this discrepancy [30].
In conclusion, the results of the current study show that a point-of-care assay can detect elevated platelet reactivity and impaired responsiveness to clopidogrel in type 2 DM patients after coronary stent implantation. The addition of cilostazol to dual anti-platelet therapy may decrease post-treatment PRU values and the frequency of HPR in patients with type 2 DM. Investigation of the clinical events associated with impaired responsiveness to clopidogrel in patients with DM is warranted.
Notes
No potential conflict of interest relevant to this article was reported.