Acute Generalized Exanthematous Pustulosis Due to Oral Use of Blue Dyes
Article information
Abstract
Acute generalized exanthematous pustulosis is a rare severe pustular cutaneous adverse reaction characterized by a rapid clinical course with typical histological findings. It is accompanied by fever and acute eruption of non-follicular pustules overlying erythrodermic skin. The causative agents are most frequently antibacterial drugs. We present a patient with acute generalized exanthematous pustulosis caused by methylene blue and indigotin dyes.
INTRODUCTION
Acute generalized exanthematous pustulosis (AGEP) is a rare and severe cutaneous hypersensitivity reaction pattern that resembles pustular psoriasis. In over 90% of cases, the causative agents are systemic drugs, particularly anti-infectious agents such as aminopenicillins and macrolides [1]. Antifungal agents, non-steroidal anti-inflammatory drugs, analgesics, antiarrhythmics, anticonvulsants, and antidepressants may also be responsible. In addition to drugs, other triggers have also been identified including exposure to mercury and viral infections [1,2].
Proposed diagnostic criteria include 1) acute development of numerous, small (< 5 mm), mainly nonfollicular pustules arising on widespread edematous erythema; 2) fever > 38℃; 3) neutrophilia with or without mild eosinophilia; 4) spontaneous resolution of pustules in < 15 days; 5) subcorneal or intraepidermal pustules on a skin biopsy [3].
We describe the first reported case of AGEP associated with blue-dye hypersensitivity.
CASE REPORT
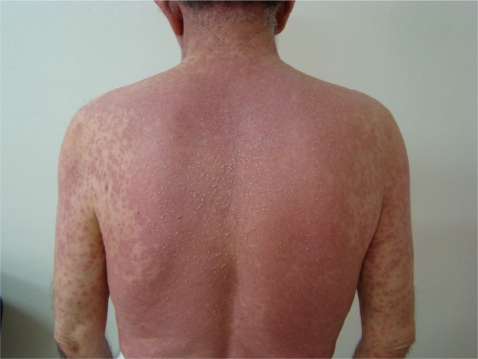
A 69-year-old male was referred to our clinic because of generalized pruritus and erythema accompanied by high fever. He had a history of hypertension for 20 years and had been taking 16 mg candesartan cilexetil tablets. The day before admission, he was given tablets three times daily for dysuria that included helmitol, methylene blue, and indigotin. On physical examination, his body temperature was 37℃. He had pruritic erythematous lesions on the trunk and upper extremities. He was diagnosed with a drug reaction due to the drug used for dysuria. The drug was discontinued, and he was given oral antihistamine therapy. After 24 hours, he was admitted with complaints of high fever (40℃), increased number of skin lesions, arthralgia, and headache. A dermatological examination revealed exanthema over 60% of the body accompanied by angioedema of the lips and eyelids. The rash was composed of scattered nonfollicular small pustules over an erythematous background (Fig. 1). No ocular or mucous membrane symptoms were present. He was clinically diagnosed with AGEP and hospitalized. A pustule culture was free of organisms. The only abnormal laboratory finding was blood eosinophilia (1.25 × 109/L). Other laboratory test results, such as a neutrophil count and serological results were within normal limits. A punch biopsy was taken from the erythematous and pustular skin area. A histopathological examination of the skin biopsy revealed subcorneal/superficial intraepidermal pustule formation and a collection of neutrophils including eosinophils. Some neutrophilic exocytosis and mild spongiosis was present around the pustules in the epidermis. The upper dermis was edematous and contained a moderate inflammatory cell infiltrate with some eosinophils in the perivascular areas (Fig. 2). The histopathological diagnosis was consistent with AGEP. He was administered an antihistamine tablet (5 mg/day levocetirizine) and both oral (48 mg/day methylprednisolone) and topical (methylprednisolone acetate) corticosteroid therapy. Omeprazol (20 mg, bid) was added to his therapy regimen. On the second day of hospitalization, he reported bloody feces.

The histopathological diagnosis was consistent with acute generalized exanthematous pustulosis. A skin biopsy showed subcorneal/superficial intraepidermal pustule formation and a collection of neutrophils, including eosinophils. (A) Superficial epidermal pustulation (H&E, × 40). (B) Neutrophilic spongiosis (H&E, × 100).
An esophagogastroduodenoscopy showed disseminated erosive gastritis of the stomach mucosa.
The pustular skin lesions and systemic findings resolved within 48 hours of hospitalization, and a second endoscopic examination was normal. Oral corticosteroid therapy was stopped on the fifth day. He continued to take his previous antihypertensive therapy (candesartan cilexetil) without any adverse reactions. He has not taken any other medications or any drug or food containing methylene blue or indigotin dye. He was discharged without antihistamine or corticosteroid therapy and invited to follow-up visits. Skin lesions and systemic symptoms had cleared up completely on day 20 after his first admission.
At the third examination 2 months after discharge, he disclosed that he had accidentally used another drug for dysuria containing helmitol. However, he did not report any adverse reactions such as those reported previously. Therefore, helmitol was omitted from the skin tests.
Skin prick tests were performed at 2 months after discharge using methylene blue and the coloring agent of the tablets, indigotin, diluted in water. The prick test was negative. Patch tests were performed with the same agent diluted to 20% in petrolatum. Patch tests were read 48 and 72 hours after application and were negative.
DISCUSSION
Methylene blue and indigotin are dyes are currently used as a tracer for detecting urinary and digestive fistulas, for assessing tubal permeability, or in sentinel lymph node biopsies [4-6].
Methylene blue is used as a treatment for some clinical conditions and for diagnostic purposes; it is an alternative choice for treating hypotension during septic shock and for treating anaphylaxis, depending on the radiocontrast material, to balance arterial blood pressure [7]. It can also be used at lower doses to treat methemoglobinemia [8].
Indigo, which is produced by fermenting the Indigofera tinctoria plant, has been used as a dye in denims, blue jeans, and other fabrics. Indigotin and indigocarmine are indigo derivatives that are widely used as synthetic coloring agents in the food and cosmetic industries in many countries. Moreover, indigocarmine is considered biologically inert and extremely safe.
Many case reports of allergic adverse reactions to blue dyes have been described. The symptoms vary from urticaria to anaphylaxis [9-11].
The physiopathological mechanisms of AGEP remain uncertain, but drug-specific positive patch test responses and in vitro lymphocyte proliferative responses in patients with a history of AGEP strongly suggest that this adverse cutaneous reaction occurs via a drug-specific T-cell-mediated process [12].
Viral infections are the cause of most drug eruptions in children, whereas drugs are more frequently responsible in adults [3]. In this case, the history and clinical course were consistent with a drug reaction; therefore, we did not conduct serological test to determine a viral etiology.
It may be clinically difficult to distinguish AGEP from other pustular dermatoses, and a histopathological examination is helpful. A histological analysis reveals subcorneal and/or intraepidermal spongiform pustulation and subcorneal collections of neutrophils with papillary dermal edema and a perivascular infiltrate consisting of neutrophils and sometimes eosinophils.
Pustules are commonly localized in the main folds (neck, axillae, and groin), trunk, and upper extremities. Additional skin symptoms, such as edema of the face and hands, purpura, vesicles, blisters, and "atypical" targets, may also be present. Mucous membrane involvement is rare, usually mild, and generally restricted to the oral mucosa. The clinical course of the skin reaction is very typical. The time interval between drug administration and skin eruption is normally less than 2 days. Skin symptoms usually resolve within a few days without treatment.
No specific treatment is recommended for AGEP except supportive care according to the clinical situation. It is a self-limited disease with a favorable prognosis.
However, AGEP may be potentially life threatening, particularly in older patients. The mortality rate of AGEP is approximately 2% [13]. Therefore, identifying and promptly removing the culprit drug is important.
Patch testing may sometimes contribute to an AGEP diagnosis; however, it did not help in this case. The sensitivity of patch testing to drugs responsible for AGEP is approximately 50% [14].
We report an interesting case of pustular eruption that presented with nonfollicular sterile pustules with diffuse edema and erythema on the face, trunk, intertriginous areas, and extremities in association with a high fever.
Although there are cases of dermatological symptoms due to blue dyes, this is the first case report of AGEP due to blue dyes [9-11]. Our AGEP diagnosis, which was aided by a skin biopsy and clinical findings, was confirmed.
Although skin epidermal prick test and patch test results did not support our diagnosis, similar negative test results have been reported in some previous cases of drug-related AGEP. This can be explained by the activation of different immunological mechanisms based on the difference in the entrance and contact of the drugs. Positive skin test results are not a typical finding of AGEP.
Notes
No potential conflict of interest relevant to this article was reported.