Feasibility of Non-TBI Conditioning with Busulfan and Fludarabine for Allogeneic Stem Cell Transplantation in Lymphoid Malignancy
Article information
Abstract
Background/Aims
This retrospective study evaluated the transplantation outcomes of patients with adult lymphoid malignancies who received chemotherapy-based conditioning with busulfan and fludarabine (BuFlu) and busulfan and cyclophosphamide (BuCy2).
Methods
Thirty-eight patients (34 with acute lymphoblastic leukemia and 4 with lymphoblastic lymphoma) were included in the current study. The conditioning regimen was BuCy2 for 14 patients and BuFlu for the remaining 24 patients. Eight and 13 patients were high risk disease in the BuCy2 and BuFlu groups, respectively.
Results
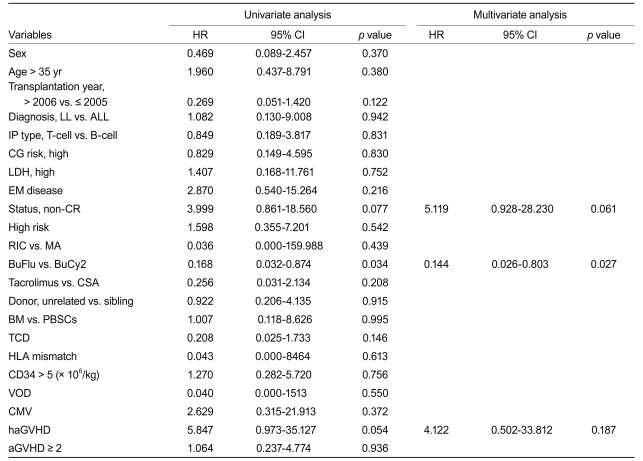
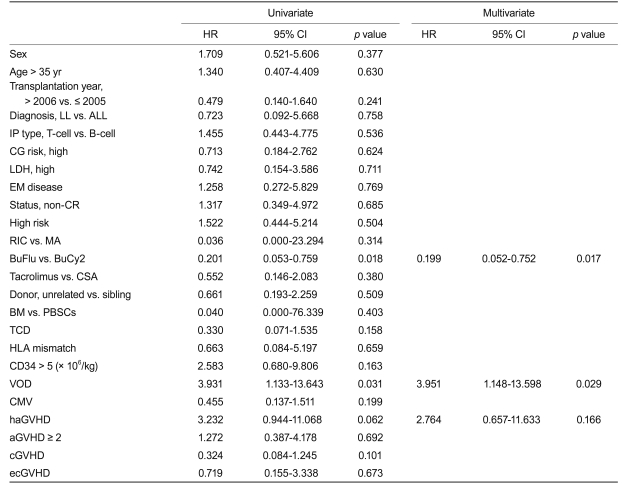
The cumulative incidence of grade II-IV acute graft-versus-host disease (GVHD) was 56.5% and 55.2% and that of extensive chronic GVHD 17.0% and 55.6% (p = 0.018) for the BuFlu and BuCy2 groups, respectively. The 3-year relapse rate was 27.8% and 31.4% and 3-year overall survival 34.3% and 46.8% for the BuFlu and BuCy2 groups, respectively. Treatment-related mortality (TRM) was significantly lower in the BuFlu group (16.9%) than in the BuCy2 group (57.1%, p = 0.010). In multivariate analyses, the BuFlu regimen was identified as an independent favorable risk factor for TRM (hazard ratio [HR], 0.036; p = 0.017) and extensive chronic GVHD (HR, 0.168; p = 0.034).
Conclusions
Our BuFlu regimen would appear to be an acceptable conditioning option for lymphoid malignancies, including high-risk diseases. It was safely administered with a lower TRM rate than BuCy2 conditioning.
INTRODUCTION
Chemotherapy-based conditioning is widely used for myeloid leukemia due to its ease of administration, reduced incidence of long-term sequelae compared to total body irradiation (TBI) conditioning, and equivalent transplantation outcomes with regard to treatment-related mortality (TRM), relapse, and leukemia-free survival (LFS) [1-5]. However, while this holds true for myeloid leukemias, there is some controversy in the case of acute lymphoblastic leukemia (ALL) due to a less potent antitumor effect at sanctuary sites [6,7].
Busulfan and cyclophosphamide (BuCy2) regimens have been standard treatments for achieving myeloablative conditioning since the publication of studies by the European Group for Blood and Marrow Transplantation (EBMT) and Center for International Blood and Marrow Transplant Research (IBMTR) that demonstrated the equivalence of BuCy2 and CyTBI regimens [1,2]. However, the major concern with BuCy2 conditioning is the high TRM caused by cyclophosphamide metabolites [8,9]. Meanwhile, fludarabine has considerable and synergistic efficacy in both immunosuppression and tumor-cell killing when administered with an alkylating agent, and is widely used as an alternative to cyclophosphamide in reduced-intensity and myeloablative conditioning [10-13].
This retrospective study evaluated the transplantation outcomes of patients with adult lymphoid malignancies who received chemotherapy-based conditioning with busulfan and fludarabine (BuFlu), adopted to avoid the sequelae of TBI conditioning and the toxicity of standard BuCy2 conditioning.
METHODS
Patients and transplantation procedures
We retrospectively reviewed the data for 38 patients with lymphoid malignancies who underwent allogeneic stem cell transplantation (SCT) at Kyungpook National University Hospital, Daegu, Korea between December 1998 and November 2009. The median follow-up after transplantation was 255 days (range, 9 to 2,804) and all of the patients received chemotherapy-based conditioning with BuCy2 or BuFlu. BuCy2 conditioning was used for patients who underwent transplantation before 2005 and BuFlu was used for those who received transplants after 2005. This study was approved by the local institutional review boards.
Until January 2008, human leukocyte antigen (HLA) typing was performed through serologic testing for class I alleles (A, B, and C) and DNA-based typing for HLA class II molecules (DRB1). However, since then, HLA-typing has been achieved through high-resolution genotyping of HLA class I and II alleles. Prophylaxis against acute graftversus- host disease (GVHD) consisted of methotrexate (MTX) plus cyclosporine for sibling donors and tacrolimus for unrelated donors. Most patients were scheduled to receive MTX (15 mg/m2 on day 1 and 10 mg/m2 on days 3, 6, and 11). The last dose of MTX was omitted when mucositis higher than grade 4 or renal impairment was observed. Cyclosporine was initially administered at a dose of 5.0 mg/kg/day via continuous infusion on the day before the transplantation. The dose was reduced to 2.5 mg/kg/day via continuous infusion on day +7, and then changed to an oral dose of 3 mg/kg twice daily when tolerated. Starting on day 60, the oral cyclosporine dose was reduced by 5% per week, and then maintained at 1.5 mg/kg/day until day 270 for patients without symptomatic chronic GVHD. Tacrolimus was administered intravenously at a dose of 0.03 mg/kg/day on the day before transplantation. Through therapeutic drug monitoring, the blood level of tacrolimus was maintained within the range of 15-20 ng/mL for the first months and 10-15 ng/mL thereafter. Patients subsequently received a daily oral dose three to four times higher than the last intravenous dose. For the purpose of in vivo T cell depletion (TCD), antithymocyte globulin (ATG) was used in patients with HLA-mismatched or unrelated donors.
The prophylactic antibiotics consisted of ciprofloxacin (250 mg, twice daily, orally), metronidazole (500 mg, three times daily, orally), and f luconazole (100 mg, once daily, orally) from the initiation of conditioning. Acyclovir (600 mg, twice daily, orally) was started from day-1 and cotrimoxazole was started after engraftment. Ursodeoxycholic acid was used for veno-occlusive disease (VOD) prophylaxis from the initiation of conditioning.
Conditioning regimens
For the myeloablative BuCy2 regimen, busulfan (Busulfex, Orphan Medical Inc., Minnetonka, MN, USA) was infused intravenously at a dose of 3.2 mg/kg/day for 4 days (total dose 12.8 mg/kg, days -7 to -4), and cyclophosphamide was infused intravenously at a dose of 60 mg/kg/day for 2 days (total dose, 120 mg/kg; days, -3 to -2). For the myeloablative BuFlu regimen, busulfan was infused intravenously at a dose of 3.2 mg/kg/day for 4 days (total dose, 12.8 mg/kg; days, -6 to -3), and fludarabine was administered intravenously at a dose of 30 mg/m2 for 6 days (total dose, 180 mg/m2/day; days, -7 to -2). For the reduced-intensity BuFlu regimen, busulfan was infused intravenously at a dose of 3.2 mg/kg/day for 2 days (total dose, 6.4 mg/kg; days, -5 to -4); the fludarabine schedule was the same as for the standard BuFlu regimen.
GVHD grading and treatment
Diagnosis and grading of acute GVHD were performed according to the consensus conference guidelines for acute GVHD [14]. The frontline treatment for acute GVHD was the prednisone (1-2 mg/kg/day). In patients with acute GVHD who did not respond to steroids and cyclosporine, cyclosporine was replaced with tacrolimus.
Chronic GVHD was diagnosed and graded based on published criteria [15]. The initial treatment for chronic GVHD was prednisone (1-2 mg/kg/day) and the reintroduction of cyclosporine or tacrolimus in the therapeutic range [16]. If further immunosuppressive agents were needed to control chronic GVHD, the salvage regimen included the use of mycofenolate mofetile (MMF), ATG, weekly MTX , or combination therapy [17,18]. MMF was added at a dose of 1.5 or 2 g/day and the steroids doses were tapered in refractory cases (by 0.2 mg/kg/wk). The dose of MMF was escalated to 2 g/day in patients with progressive GVHD.
Definitions
The day of stem cell infusion was defined as day 0. Myeloid engraftment was defined as the first day of a period of at least three consecutive days with an absolute neutrophil count (ANC) of ≥ 0.5 × 109/L, while platelet engraftment was defined as the first day of at least three consecutive days on which a platelet count of ≥ 20 × 109/L was achieved without transfusion. High-risk patients were defined as patients older than 35 years or those with a high white blood cell count at presentation (≥ 100 × 109/L for B-lineage cells and ≥ 30 × 109/L for T-lineage cells), along with all patients with Philadelphia chromosome. TRM was defined as mortality related to stem cell transplantation procedures, e.g., VOD, GVHD, and infection.
Statistical analysis
Continuous variables were compared using the two-sample t test, while categorical data were analyzed using the chi-square test. Overall survival (OS) was defined as the time from transplantation until death from any cause, and was analyzed using a Kaplan-Meyer test. Both groups were compared using a log-rank test or Breslow test. The cumulative incidence of chronic GVHD was calculated by Gray's method (with death or relapse without chronic GVHD considered as competing risks) using the R software package cmprsk. A time-dependent Cox regression model was used to identify clinical predictors of the development of chronic GVHD and TRM. Factors with p values of 0.1 or less in the univariate analysis were included in the multivariate analysis and the variables were analyzed using backward inclusion methods. For the statistical analyses, SPSS version 13 (SPSS Inc., Chicago, IL, USA) and R statistical software version 2.8.0 (R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org) were used. A p value of < 0.05 was considered significant.
RESULTS
Patient characteristics
Thirty-four patients were diagnosed with ALL and four with lymphoblastic lymphoma. The conditioning regimen was BuCy2 for 14 patients and BuFlu for 24 patients. Six patients underwent reduced-intensity BuFlu conditioning (RIC). Median age in the BuCy2 and BuFlu groups was 31 years (range, 18 to 48) and 33 years (range, 22 to 53), respectively. There were 13 (54.2%) and 8 (57.1%) highrisk patients in the BuCy2 and BuFlu groups, respectively, including 1 (7.1%) and 7 (29.2%) Ph-chromosome-positive cases, respectively (p = 0.859). Initial extramedullary involvement was present in four (28.6%) and two (2.8%) patients in the BuCy2 and BuFlu groups, respectively (p = 0.099). The pretransplantation procedures were comparable regarding HLA-mismatched donors (7.1% vs. 16.7%, p = 0.370), unrelated donors (28.6% vs. 58.3%, p = 0.076), and peripheral blood stem cells (85.7% vs. 91.7%, p = 0.564). The other patient characteristics are summarized in Table 1.
Survival
The survival rate for all patients at 3 years was 41.8% (95% confidence interval [CI], 27.5 to 63.4) with a median follow-up duration of 982 days (95% CI, 634 to 1,329). The 3-year OS rate was 53.8% (95% CI, 33.7 to 86.0) for standard-risk patients and 30.6% (95% CI, 14.3 to 65.1) for the high-risk patients (p = 0.213) (Fig. 1), while the 3-year relapse rate was 14.7% for the standard-risk patients and 43.3% for the high-risk patients (p = 0.109). Patients with chronic GVHD had a better 3-year OS rate (64.0%) than those without chronic GVHD (24.0%, p = 0.002) (Fig. 1). The 3-year OS for the BuCy2 and BuFlu groups was 34.3% and 46.8%, respectively (p = 0.279) (Fig. 2), while the 3-year event-free survival (EFS) rate was 25.7% and 47.1%, respectively (p = 0.215) (Fig. 2). Relapse occurred in three patients (21.4%) in the BuCy2 group and six patients (25.0%) in the BuFlu group (p = 0.803) (Table 2). The cumulative incidence of 3-year relapse for the BuCy2 and BuFlu groups was 27.8% and 31.4%, respectively (p = 0.476) (Fig. 3).
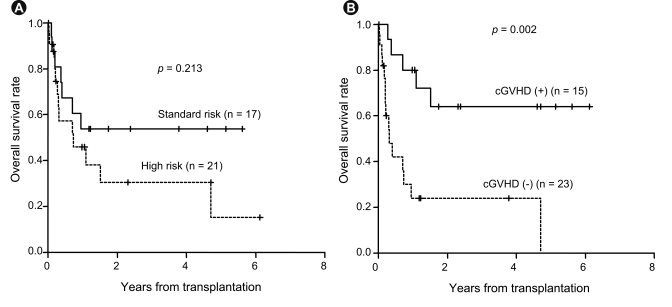
Survival rates according to risk group and presence of graft-versus-host disease (GVHD). (A) Overall survival according to risk group. The 3-year overall survival (OS) rate was 53.8% (95% confidence interval, 33.7 to 86.0) for standard-risk patients and 30.6% (95% confidence interval, 14.3 to 65.1) for high-risk patients. (B) Overall survival according to presence of chronic GVHD. Patients with chronic GVHD showed a better 3-year OS rate (64.0%) than those without chronic GVHD (24.0%).
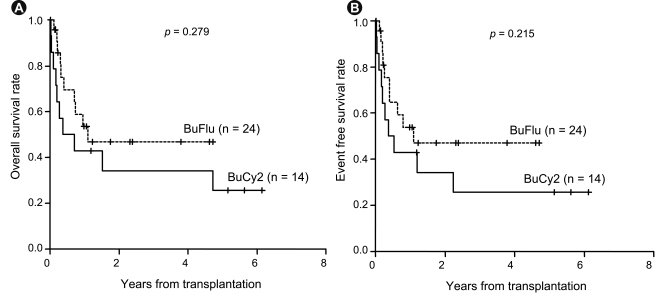
Survival analyses according to conditioning regimen. (A) Overall survival. The 3-year overall survival rates for the busulfan-cyclophosphamide (BuCy2) and busulfan-fludarabine (BuFlu) groups were 34.3% and 46.8%, respectively. (B) Event-free survival. The 3-year event-free survival rates for the BuCy2 and BuFlu groups were 25.7% and 47.1%, respectively.
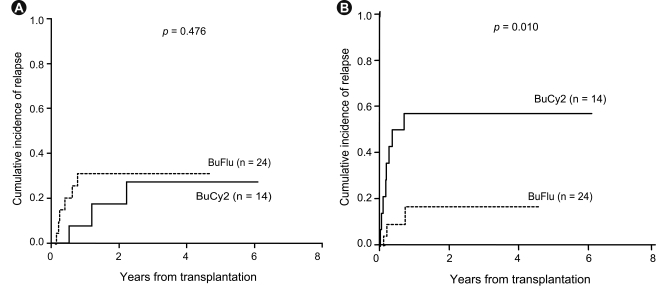
Relapse and treatment-related mortality (TRM) according to conditioning regimen. (A) Cumulative incidence of relapse. The cumulative incidence of 3-year relapse for the busulfan-cyclophosphamide (BuCy2) and busulfan-fludarabine (BuFlu) groups was 27.8% and 31.4%, respectively. (B) Treatment-related mortality. The cumulative incidence of trm was significantly lower for the BuFlu group (16.9%) than for the BuCy2 group.
Graft-versus-host disease
The incidence of acute GVHD did not differ between the two groups. Grade II-IV acute GVHD developed in 7 (50.0%) of the 14 patients in the BuCy2 group at a median of 15 days (range, 9 to 22) and in 7 (29.2%) of the 24 patients in the BuFlu group at a median of 24 days (range, 12 to 64) (p = 0.199) (Table 2). The cumulative incidence of grade II-IV acute GVHD 100 days after transplantation was 56.5% in the BuCy2 group and 55.2% in the BuFlu group (p = 0.130) (Fig. 4). Among patients who survived longer than 3 months after transplantation, limited and extensive chronic GVHD developed in two (22.2%) and five (55.6%) patients, respectively, in the BuCy2 group, and in six (35.3%) and two (11.8%) patients, respectively, in the BuFlu group (p = 0.054) (Table 2). The cumulative incidence of extensive chronic GVHD after transplantation in the BuFlu group (17.0%) was lower than that in the BuCy2 group (55.6%; p = 0.018) (Fig. 4).
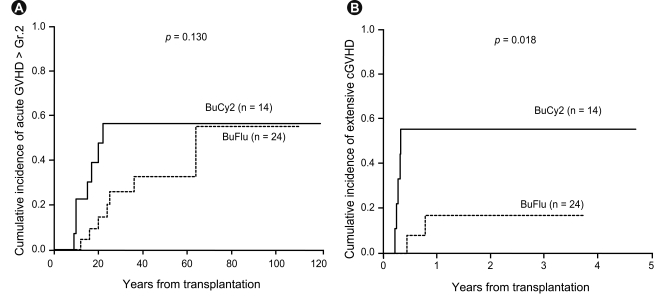
Graft-versus-host disease (GVHD) according to conditioning regimen. (A) Cumulative incidence of grade II-IV acute GVHD. The cumulative incidence of grade II-IV acute GVHD at 100 days after transplantation was 56.5% for the busulfan-cyclophosphamide (BuCy2) group and 55.2% for the busulfan-fludarabine (BuFlu) group. (B) Cumulative incidence of extensive chronic GVHD. Among patients who survived longer than 3 months after transplantation, the cumulative incidence of extensive chronic GVHD after transplantation was lower in the BuFlu group (17.0%) than in the BuCy2 group (55.6%).
In univariate analyses, complete remission (CR) (hazard ratio [HR], 3.999; p = 0.077), the BuFlu regimen (HR, 0.168; p = 0.034), and hyperacute GVHD (HR, 5.847; p = 0.054) were identified as factors affecting TRM. Meanwhile, multivariate analysis identified the BuFlu regimen (HR, 0.144; 95% CI, 0.026 to 0.803; p = 0.027) as an independent favorable risk factor for the development of extensive chronic GVHD (Table 3).
Treatment-related mortality
TRM affected eight patients (57.1%) in the BuCy2 group and three patients (12.5%) in the BuFlu group (p = 0.008) (Table 2). The cumulative incidence of TRM was significantly lower in the BuFlu group (16.9%) than in the BuCy2 group (57.1%, p = 0.010) (Fig. 3). Causes of TRM in the BuCy2 group included infection (two patients, 14.3%), chronic GVHD (two, 14.3%), VOD (two, 14.3%), and brain hemorrhage (two, 14.3%); in the BuFlu group, they included infection (two patients, 8.3%) and VOD (one, 4.2%).
In univariate analyses, the BuFlu regimen (HR, 0.201; p = 0.018), VOD (HR, 3.931; p = 0.031), and hyperacute GVHD (HR, 3.232; p = 0.062) were identif ied as significant factors affecting TRM. Multivariate analysis identified the BuFlu regimen (HR, 0.199; 95% CI, 0.052 to 0.752; p = 0.017) as an independent favorable risk factor for TRM, and VOD (HR, 3.951; 95% CI, 1.148 to 13.598; p = 0.029) as an unfavorable risk factor for TRM (Table 4).
Extramedullary disease
Among the six patients who presented with extramedullary involvement at the time of diagnosis, the five with positive cerebrospinal fluid cytology were treated with intrathecal chemotherapy, while the remaining patient, who had a central nervous system (CNS) mass, was treated with brain radiotherapy and intrathecal chemotherapy (Table 5). All six patients were treated successfully with no CNS system relapse, although four of them experienced bone marrow relapse and the patient with the CNS mass developed a pelvic mass, which was successfully treated with radiotherapy and dasatinib treatment. Extramedullary relapse was observed in two of the nine patients who relapsed. Two of the thirty-two patients without extramedullary involvement at presentation experienced recurrence in the CNS as well as the BM, even though they had received prophylactic CNS treatment. However, both of these patients presented with complex cytogenetic abnormalities at the time of diagnosis and did not show any evidence of chronic GVHD after transplantation.
DISCUSSION
Many studies have already investigated substituting busulfan for TBI to reduce the long-term side effects of TBI, and randomized studies comparing TBI-based and non-TBI-based regimens have shown comparable results for myeloid leukemias. However, experience with chemotherapy-based regimens is limited, especially for adult patients with ALL. Moreover, there have been conflicting outcomes [1-5].
In the current study, when using non-TBI-based conditioning, the survival rate was comparable to that reported in previous studies, with an overall 3-year OS rate of 41.8% (53.8% for the standard-risk patients and 30.6% for the high-risk patients) (Fig. 1). Meanwhile, patients with chronic GVHD showed a better survival rate than those without chronic GVHD, demonstrating the strong anti-leukemia effect of GVHD. These results are supported by the results of the subgroup analysis in the EBMT trial, which found no significant differences between patients treated with BuCy2 and those treated with CyTBI in terms of TRM, the incidence of relapse, or LFS [1]. LFS among allograft recipients with intermediaterisk ALL was 43 ± 6% in the BuCy2 group and 33 ± 6% in the CyTBI group, a difference that was not statistically significant. However, there remains some controversy over the role of non-TBI conditioning in ALL patients.
BuCy2 has been used in adult patients with ALL and has an LFS rate that is comparable to those of radiation-containing regimens [19]. In contrast, in a study conducted by Granados et al. [20], TBI was associated with a lower relapse rate and better EFS than a busulfan-based conditioning regimen. However, it is difficult to compare the results directly because Granados et al. [20] included some childhood ALL cases, which might have affected the outcomes, and used oral busulfan instead of IV busulfan. The inferior survival rate for BuCy2 patients, especially children, was likely due to the oral busulfan used in these trials. The intestinal absorption of oral busulfan is unpredictable, causing inter-patient variability in the plasma concentration. Moreover, the total plasma clearance rate is two to four times higher in children than in adults [21-25]. In contrast, intravenous preparations of busulfan produce a more predictable steady-state concentration, resulting in a lower incidence of hepatic VOD and better 100-day survival [26].
Experience with fludarabine-containing conditioning for adult ALL patients is very limited. In a study by Iravani et al. [13] using a myeloablative BuFlu conditioning regimen, the 1-year OS for ALL patients was 55.6%, with a 33.3% relapse rate at 1 year. In the current study, the outcomes for BuFlu conditioning were comparable to those for BuCy2 in terms of OS, EFS, and incidence of relapse (Figs. 2 and 3). The cumulative incidence of extensive chronic GVHD was significantly lower in the BuFlu group than in the BuCy2 group. Indeed, the BuFlu regimen was an independent favorable risk factor for the development of extensive chronic GVHD. In addition, while the higher relative incidence of TRM remains a major concern for patients receiving BuCy2 conditioning [12,13], this was not a significant issue for patients who received BuFlu conditioning (Table 4). However, interpretation of the current results requires caution because the disease status before transplantation was different in the two groups and, as BuFlu conditioning was performed more recently, improvements in supportive care may have contributed to the favorable outcome regarding TRM in the BuFlu group. CR1 was achieved in more patients in the BuFlu group (75.0%) than in the BuCy2 group (42.9%). Moreover, although only a small number of patients with the Philadelphia chromosome were included in the current study, tyrosine kinase inhibitors were used in more patients in the BuFlu group. In terms of conditioning intensity, RIC in the BuFlu group also confounded the difference in incidence of GVHD between the groups [27]. In addition, to achieve more meaningful results requires analysis of the data after distinguishing between sibling and unrelated donors and a larger sample size.
The major concern with chemotherapy-based conditioning in ALL is overcoming the blood-brain barrier. Bunin et al. [6] reported 5 cases of extramedullary relapse (23.8%) with BuCy2 and 2 cases of testicular relapse (9.1%) with TBI, but only 1 case of CNS relapse out of 52 patients who followed a BuCy2 regimen, this patient having previously received cranial irradiation to treat multiply relapsed CNS leukemia [6]. Meanwhile, in the present study, extramedullary relapse in the CNS was only observed in 2 of the 32 patients without extramedullary involvement at presentation, and both of these patients had complex cytogenetic abnormalities at presentation and exhibited no evidence of chronic GVHD after transplantation. Recent studies have indicated that the graft-versus-leukemia (GVL) effect may play a significant role in preventing CNS relapse in patients with lymphoid malignancies who only receive chemotherapy-based conditioning for allogeneic transplantation [28]. In the international collaborative trial conducted by Goldstone et al. [29] (MRC UKALL XII/ECOG E2993), the 5-year OS rate for adult ALL patients was 53% for standardrisk patients and 41% for high-risk patients, while the 5-year relapse rate was 24% for standard-risk patients and 37% for high-risk patients, leading to speculation that allogeneic transplantation has the most potent antileukemic effect in adult ALL, as demonstrated by the significantly reduced relapse rate (37% with a donor vs. 63% without a donor, p < 0.001).
In conclusion, our BuFlu regimen would appear to be an acceptable conditioning option for lymphoid malignancies, including high-risk cases, in terms of OS and EFS. It was safely administered with a lower TRM rate compared to BuCy2 conditioning. When considering the graftversus-leukemia effect, sanctuary site relapse after transplantation would not seem to be a significant issue in patients with lymphoid malignancies.
Notes
No potential conflict of interest relevant to this article was reported.