Endoscopic drainage in patients with inoperable hilar cholangiocarcinoma
Article information
Abstract
Hilar cholangiocarcinoma has an extremely poor prognosis and is usually diagnosed at an advanced stage. Palliative management plays an important role in the treatment of patients with inoperable hilar cholangiocarcinoma. Surgical, percutaneous, and endoscopic biliary drainage are three modalities available to resolve obstructive jaundice. Plastic stents were widely used in the past; however, self-expanding metal stents (SEMS) have become popular recently due to their long patency and reduced risk of side branch obstruction, and SEMS are now the accepted treatment of choice for hilar cholangiocarcinoma. Bilateral drainage provides more normal and physiological biliary flow through the biliary ductal system than that of unilateral drainage. Unilateral drainage was preferred until recently because of its technical simplicity. But, with advancements in technology, bilateral drainage now achieves a high success rate and is the preferred treatment modality in many centers. However, the choice of unilateral or bilateral drainage is still controversial, and more studies are needed. This review focuses on the endoscopic method and discusses stent materials and types of procedures for patients with a hilar cholangiocarcinoma.
INTRODUCTION
Cholangiocarcinoma is a cancer that originates from the bile duct epithelium and is classified as intrahepatic, hilar, or extrahepatic depending on its location. Hilar cholangiocarcinoma was first described by Altemeier et al. [1] in 1957. However, it was only after a study of a series of 13 patients in 1965 that this tumor was recognized as a distinct clinical entity and was named for Dr. Gerald Klatskin [2].
Resection with negative margins is the only available curative treatment for hilar cholangiocarcinoma. However, most patients with hilar cholangiocarcinoma display advanced disease, metastasis, and co-morbidity at the time of presentation and have an extremely poor prognosis, with less than 10% of patients surviving 5 years after diagnosis. Palliative management should be considered in these patients. The aims of palliation in patients with unresectable hilar cholangiocarcinoma according to the British Association for the Study of Liver are to improve the quality of life by relieving obstructive jaundice, pruritus, cholangitis, or pain, and secondarily, to prolong survival. Currently available modalities for palliation of unresectable hilar cholangiocarcinoma are surgical, percutaneous, and endoscopic drainage. An ideal palliative procedure should be simple and effective to relieve obstructive cholestasis, have low procedure-related morbidity and mortality, and offer durable palliation. Until now, there has been no clear consensus concerning which is the best procedure for biliary drainage of hilar cholangiocarcinoma. Endoscopic biliary drainage (EBD) has assumed an increasingly important role in the palliation of patients with unresectable hilar cholangiocarcinoma because of its efficacy, patient acceptance, and relatively low morbidity and mortality.
The two types of EBD are endoscopic nasobiliary drainage (ENBD) and endoscopic retrograde biliary drainage (ERBD). ENBD is an endoscopic procedure that uses a 250-cm long, preformed polyethylene tube placed above the stenosis in an obstructed biliary tract to provide flow out of the bile [3]. The presence of an external tube through the nose is uncomfortable and, thus, is temporarily used in patients awaiting surgery or evaluation. ERBD is the preferred choice for palliation of patients with hilar cholangiocarcinoma because it offers the advantages of physiological bile drainage and increased patient comfort.
Although EBD has been widely used with either plastic or metal stents, the choice of optimal treatment modality in unresectable hilar cholangiocarcinoma is complicated by the requirement of a higher degree of expertise and the considerably higher morbidity and mortality rates. The use of plastic versus metal stents and a single versus multiple stents for endoscopic palliation is controversial.
This review will focus on the endoscopic method and discuss stent materials and types of procedures.
PLASTIC VERSUS METAL STENTS
Plastic stents
The plastic stent has been used for EBD for more than three decades. Several studies have suggested that stents of 10- and 12-Fr diameters are superior in terms of patency compared to those with a 7-Fr diameter [4,5]. The average duration of patency is 3 to 4 months [6]. Therefore, a plastic stent diameter of at least 10 Fr is recommended for effective drainage for a particular period of time [5].
Plastic stents have advantages that include low cost, simple insertion, and easy removal. Plastic stents have been used to achieve biliary drainage in patients with a malignant hilar obstruction. However, the use of plastic stents for hilar cholangiocarcinoma has revealed poor results in some series [7-9]. Liu et al. [10] studied the usefulness of endoscopic insertion of plastic stents in 55 patients with hilar cholangiocarcinoma, and endoscopic stenting was attempted in 49 patients. Technical success rate, clinical success rate, and early complication rate were 73%, 41%, and 25%, respectively. The median patency of the first stent was just 1 week (range, 0 to 8). Stent patency was also relatively short (49 days) in another study [11].
The main disadvantage of plastic stents is their ease of clogging. Stent occlusion frequently results in cholangitis with rates as high as 20% to 40% for unilateral or bilateral plastic stents. Clogging is caused by a relatively narrow lumen (10 to 12 Fr) and is proportional to stent length [7]. The mechanism of stent clogging is multi-factorial and is associated mainly with bacterial contamination of undrained bile ducts [8,9]. Another disadvantage of plastic stents is the technical limitation of inserting a large diameter plastic stent. It is technically difficult to place more than one 10-Fr stent at the initial setting. Moreover, plastic stents in the hilum are prone to distal migration.
Due to the plastic stent clogging problem, stent exchange is required in one third of patients [12]. It is necessary to exchange plastic stents regularly every 3 to 4 months or when obstructive symptoms appear [13]. Many trials to prevent stent clogging, including increasing stent diameter, prophylactic administration of antibiotics, administration of drugs such as ursodeoxycholic acid that alter bile composition, and using newly modified stents have been tried, but most results have been disappointing [14-18]. Only increased stent diameter has prolonged stent patency [19,20]. Although no randomized controlled studies have compared plastic and metal stents, plastic stent use in patients with hilar cholangiocarcinoma has decreased because of its relatively short patency, difficulty of multiple stenting, and obstructive risk of side branches.
Metal stents
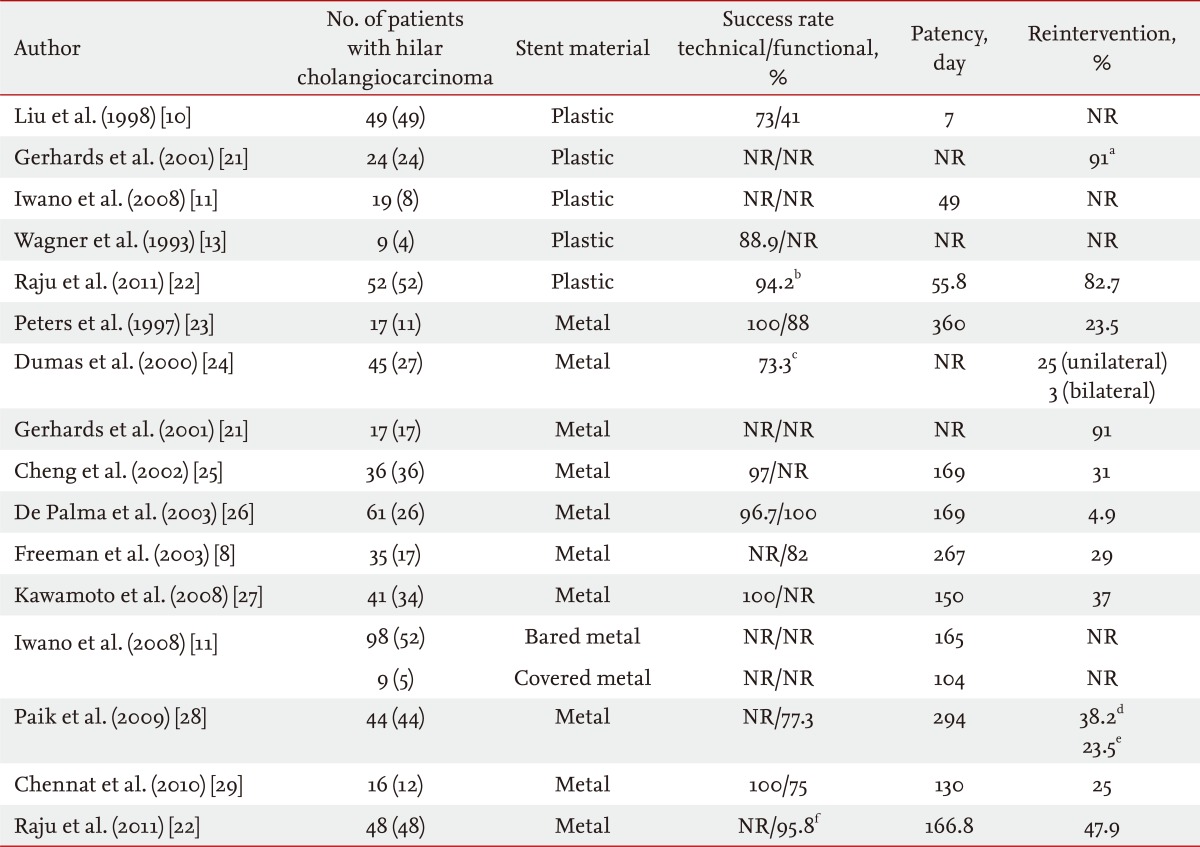
Self-expanding metal stents (SEMS) were developed to overcome the limitations of the plastic stent. The main advantage of SEMS is their large internal diameter (generally 10 mm; i.e., 30 Fr), and they provide patency duration of about 10 months, reducing the necessity for reintervention (Table 1) [8,10,11,13,21-29]. SEMS also allows drainage of secondary branches through the open side mesh of the stent. In a study comparing plastic and metal stents in 20 patients with hilar cholangiocarcinoma (Bismuth II to IV), SEMS showed higher patency rates, a lower rate of cholangitis (33.3% vs. 9.1%), and required fewer reinterventions (2.4 ± 2.6 vs. 0.4 ± 0.5). Median patency was not described accurately, but stent malfunction was observed in 50% of patients in the plastic group and 18.2% in the metal group after long-term follow-up (> 30 days) [13].
Raju et al. [22] compared plastic and metal stents in 100 patients with hilar cholangiocarcinoma. Metal and plastic stents were placed in 52 and 48 patients, respectively. Successful drainage was achieved in 46 (95.8%) in the SEMS group and 49 (94.2%) in the plastic group. Median patency was 5.56 months in the SEMS group and 1.86 months in the plastic group. Median survival was 9.08 and 8.22 months in the SEMS and plastic groups, respectively. More reintervention was needed in the plastic group [22].
Moss et al. [5] reported that metal stents are associated with a significantly lower relative risk (0.44) of stent occlusion than that of plastic stents. The median increase in the cost effectiveness ratio of metal stents is US $1,820 per endoscopic retrograde cholangiopancreatography (ERCP) prevented [6]. Another study reported that even in countries where the cost of the ERCP procedure are lower than those of SEMS, the average total cost is lower for the SEMS group compared with that in the plastic group [30].
Although their initial cost is higher, SEMS have been increasingly used based on the above results. Compared with plastic stents, a larger diameter SEMS results in improved stent patency and fewer repeat procedures [9,31,32]. In addition, the open-mesh design allows drainage of secondary branches through the side of the stent and less likelihood for obstructive segmental cholangitis. Insertion of an uncovered SEMS is preferred when unilateral drainage is planned due to prolonged patency and prevention of branch duct obstruction. Although few data are available on hilar cholangiocarcinoma, current evidence favors placement of SEMS as they provide superior palliation in comparison to plastic stents in terms of early and late complications [23,33].
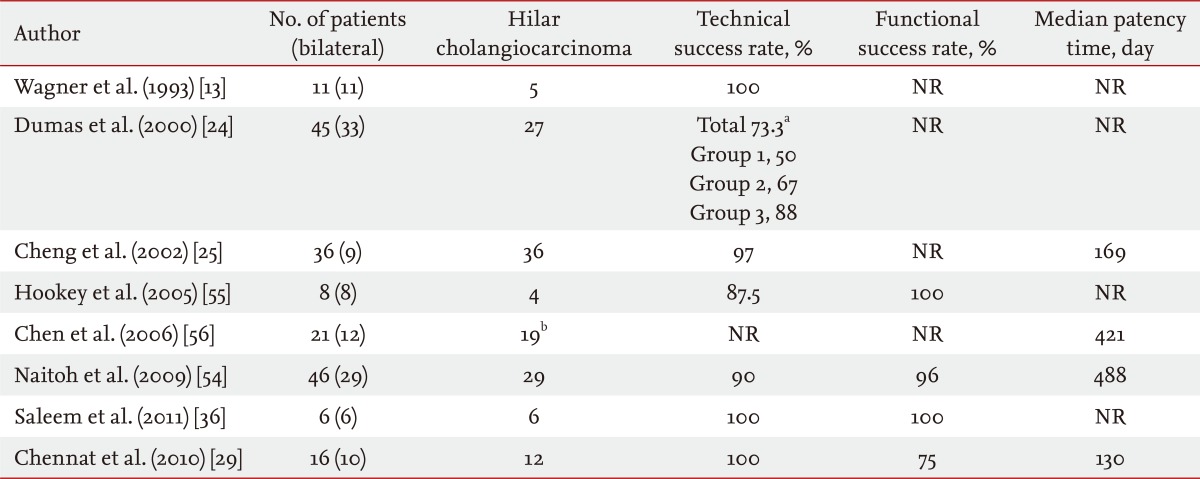
The main problem with uncovered SEMS is tumor in growth into the stent lumen, which results in stent obstruction. Polyurethane or silicone covered SEMS have been developed to prevent tumor in-growth. The results of some studies support this hypothesis, but contradictory results have also been reported [34-37]. Another study performed with 65 patients with hilar cholangiocarcinoma compared 19 plastic stents, 98 bare metal stents, and nine covered metal stents. The patencies of the plastic stent, bare metal stent, and covered metal stent were 49, 165, and 104 days, respectively. The median survival time and median event-free survival time were also longer in the bare metal stent group than those in the plastic stent group. Although that study was limited by the heterogeneity of the patient groups and types of stents, the patency of the covered metal stent seemed not to be superior to that of the bare metal stent. A covered stent is not usually used in patients with hilar cholangiocarcinoma due to unintentional obstruction of contralateral ducts or side branch ducts and lack of improved stent patency. Thus, uncovered SEMS is the treatment of choice in patients with hilar cholangiocarcinoma.
UNILATERAL VERSUS BILATERAL STENTS
Adequate palliation of obstructive cholestasis is achieved by draining only 25% of the liver volume [38]. Inserting a single biliary stent into one functional liver lobe for unilateral drainage can provide adequate palliation in the majority portion of patients with hilar tumors [39].
Some clinicians agree that placing a unilateral stent is sufficient to provide adequate drainage and has lower morbidity due to the ease of the procedure and fewer complications compared to those of bilateral stents [23,40-44]. De Palma et al. [44] reported that inserting a unilateral stent is associated with a significantly higher stent insertion success rate, higher rate of successful drainage, and lower incidence of early complications including cholangitis. No differences were observed with regard to late complications and survival. Although very limited data are available comparing unilateral with bilateral SEMS in unresectable hilar obstructions, those available indicate that unilateral biliary stenting is sufficient for Bismuth type I to IV cases [26,44-46]. Five major studies compared unilateral and bilateral endoscopic stenting in patients with malignant hilar obstruction, and the results were conflicting [33,44-47].
A higher incidence of cholangitis, 30-day mortality, and short survival duration have been reported in patients treated with unilateral drainage [47]. Reintervention is frequently necessary due to stent occlusion caused by debris and food occlusion, tumor in growth, tumor over growth, and stent migration [48].
Several studies have attempted to address whether unilateral or bilateral drainage is effective for palliative treatment of obstructive jaundice in patients with hilar cholangiocarcinoma [33,44,47,49]. Inserting bilateral stents is associated with an increased survival rate and reduced risk of cholangitis, compared to that of unilateral drainage for Bismuth types II and III hilar tumors [47]. The highest survival rate is noted in patients with hilar tumors who have bilateral drainage, whereas the lowest survival rate is evident in those who show cholangiographic opacification of both lobes but only unilateral drainage was available [33]. However, an unsuccessful attempt at bilateral drainage can lead to increased incidence of postprocedural cholangitis and lower survival rates [42,44]. Therefore, palliation of hilar obstructions should be undertaken only in highly regarded institutions with high success rates for drainage of hilar obstructions.
Although there are advantages to bilateral drainage and disadvantages to unilateral drainage, endoscopists hesitate to place bilateral biliary stents because of the inherent technical difficulties that result in lower success rates and higher complication rates compared with those of unilateral stent insertion [26]. The necessity to drain both liver lobes is still questioned and controversial in Bismuth type II to IV strictures due to a paucity of data from randomized controlled trials of the usefulness of bilateral stenting.
Bilateral drainage theoretically provides more normal and physiological biliary flow through both ductal systems into the common bile duct than that of unilateral drainage [45-47,50]. In addition, a recent study of the effective volume of liver lobe drainage (hepatic volume assessed by cross-sectional imaging) supported the use of bilateral or multiple drainage. The main factor associated with drainage effectiveness is a drained liver volume > 50%, which frequently requires bilateral stent placement and which is associated with a longer median survival (119 days vs. 59 days) in patients with hilar cholangiocarcinoma [51]. Bulajic et al. [52] also assumed that ≥ 50% drainage of liver volume leads to sufficient drainage. Both these studies favored bilateral drainage.
Many centers now prefer bilateral drainage with advancements in technology because of the potentially increased risks of cholangitis and complications associated with unilateral drainage [33,38,47,53,54]. Bilateral drainage may provide increased functional liver volume and prevent possible complications during future chemotherapy. In addition, bilateral biliary drainage is necessary when unilateral drainage cannot relieve jaundice or when initial unilateral drainage is complicated by contralateral cholangitis.
Two recent studies reported results comparing bilateral and unilateral drainage. Naitoh et al. [54] retrospectively reviewed 46 patients including 22 with hilar cholangiocarcinoma and a malignant hilar obstruction. The median stent patency rate was significantly higher in the bilateral SEMS stenting group (n = 17; 448 days) compared with that in the unilateral group (n = 29; 210 days). No significant difference was observed in successful stent insertions, successful drainage, early or late complications, or survival between the unilateral and bilateral groups [54]. Bulajic et al. [52] comcompared unilateral versus bilateral drainage in patients with hilar malignant strictures (n = 49, including 32 hilar cholangiocarcinomas). The unilateral group (n = 21) and the bilateral group (n = 18) were not different in terms of early complication rates or survival time. However, clinical success and late complications were significantly different, and favored the bilateral group [52].
TECHNICAL ASPECTS OF BILIARY STENTING
Unilateral stenting
Unilateral stenting is simple and similar to the conventional endoscopic stenting technique. An unresolved issue concerns which duct should be selected and drained when placing a unilateral stent. A developing approach to manage malignant hilar strictures is the use of magnetic resonance cholangiopancreatography (MRCP) or computed tomography (CT)-guided unilateral endoscopic drainage [42]. This technique involves obtaining an MRCP and/or CT image before the endoscopic intervention. MRCP/CT images are useful to determine the main hepatic duct that drains the largest number of viable segments, which avoids draining atrophied lobes [41,42,49]. The postprocedural cholangitis risk can be reduced by avoiding drainage of atrophied lobes. However, it is very difficult to select the intended duct endoscopically compared to the percutaneous route. Other authors have suggested no difference whether the right or left hepatic duct is drained, and that the most easily accessible duct should be used [45]. However, the right hepatic duct is frequently and more easily accessed anatomically, regardless of obstruction severity. A mildly obstructed duct that drains well and has less necessity for drainage is frequently selected and drained, instead of an obstructed duct or a duct causing cholangitis.
Bilateral stenting
Bilateral drainage is technically difficult and challenging. Sphincterotomy is always performed with the use of prophylactic antibiotics. Care must be taken during the initial positioning of every guidewire to ensure that the left-sided guidewire is inserted first if possible because left stenting is more difficult to achieve than right stenting. When it is very difficult to initially place the guidewire into the left hepatic duct, "vice versa" is an alternative. "Stent by stent" and "stent in stent" are the two techniques available for endoscopic bilateral stenting. The "stent-by-stent" technique is a bilateral stenting procedure with parallel SEMS to drain both intrahepatic ducts. The technique is performed as follows: 1) initial insertion of bilateral semi-rigid hydrophilic guidewires with flexible tips, 2) ensuring that the guidewires are not entangled, 3) preliminary bilateral dilation of the stenosis using a hydrostatic balloon, and 4) initial insertion of the left-sided stent followed by insertion of the right-sided stent (Fig. 1). Several studies have reported endoscopic bilateral biliary drainage using this technique (Table 2) [13,24,25,29,36,54-56].
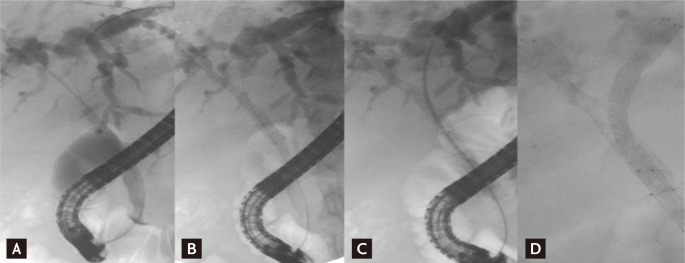
"Stent-by-stent" method. (A) Guidewires are placed in both intraheptic ducts (IHDs). (B) The first stent is placed in the right IHD. (C) The undeployed stent is introduced into the left IHD. (D) Both stents are deployed in a parallel arrangement.
The "stent-by-stent" technique has some problems and difficulties that include potential entanglement of the two guidewires, fragility and dislodgement of the stents, and difficulty positioning both stents exactly. As an alternative, the "stent-in-stent" technique was developed to place bilateral SEMS in a Y configuration, in which the second stent traverses through the open-mesh wall of the first stent to enter the contralateral bile duct [57]. The transverse stent with the Y configuration, called a Y stent (Niti-S Biliary Y stent, Taewoong, Seoul, Korea), was developed as a hybrid of the spiral and Z components (Fig. 2).
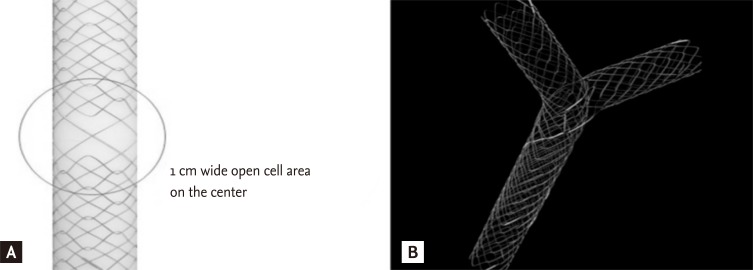
Configuration of the Y stent. (A) Central wide open-mesh portion. (B) Y configuration when placed bilaterally.
The mesh of the central part of the Y stent becomes bigger by omitting the Z component (forming a central area of relatively wide-open mesh), resulting in an open-weave portion that is 10 mm long in the middle of the stent (the present form is composed of a longer central wide portion, whole large cell type, or open mesh design). The Y stent is 6 to 8 cm in length and 10 mm in diameter, with gold markers indicating the central mesh. The first Y stent has two major disadvantages: the tendency for easy tumor in-growth in the wider-central mesh portion compared to that of the smaller mesh portions on both ends, and poor stent expansion due to weak radial force. Several improved SEMS versions have been developed to overcome the disadvantages of the first Y stent (M-Hilar stent, Standard Sci-Tech, Seoul, Korea and LCD type Y stent, Taewoong, respectively). Several types of second stents (Niti-S, Niti-D, LCD type stent, Taewoong; M-Hilar stent, Standard Sci-Tech; Zilver stent, Cook Endoscopy, Winston-Salem, NC, USA), introduced via the central mesh of the Y stent, have a length of 6 to 8 cm and a diameter of 10 mm. The stent placement technique is as follows. After the strictures are negotiated with the guidewire into the left hepatic duct, the Y stent is deployed in the left hepatic duct. The guidewire left in place across the Y stent is carefully withdrawn with an ERCP catheter, without pulling it back completely, and is then inserted under fluoroscopic guidance into the right hepatic duct through the central open mesh of the Y stent. Another uncovered second metal stent is then introduced over the guidewire through the central open mesh. This stent is deployed in the right hepatic duct (Fig. 3).
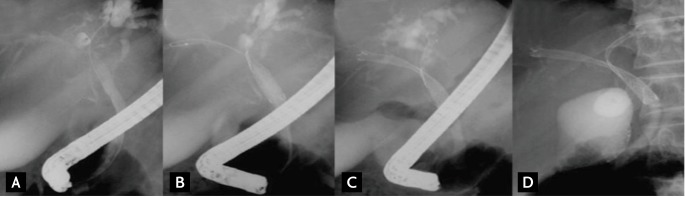
"Stent-in-stent" method. (A) Guidewire is introduced into the left intrahepatic duct (IHD). (B) Y stent is placed in the left IHD. Guidewire is introduced into the right IHD through the central open-mesh of the Y stent. (C) The second stent is deployed in the right IHD. (D) Y configuration bilateral stenting accomplished.
When difficulties are encountered when inserting the guidewire into the intended hepatic duct, modified techniques involve using a more hydrophilic Terumo guidewire, a stiffer guidewire, a retrieval balloon catheter, a pull-type endoscopic sphincterotomy knife, or a triple-lumen catheter (Haber RAMP catheter, Cook Endoscopy) [24,29,42,54,58,59].
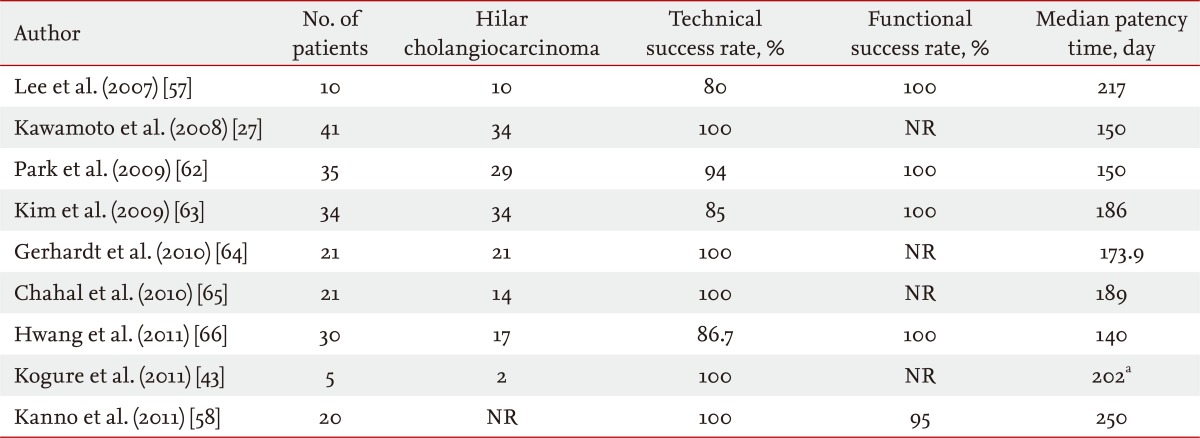
Passing a tapered-tip catheter or a stent through the stricture can be unsuccessful, even after obtaining guidewire access to the desired hepatic duct. In these cases, a 7-Fr Soehendra stent extractor (Cook Endoscopy), a 6 to 8 mm hydrostatic balloon (Hurricane CRE wire-guided esophageal/pyloric balloon dilation catheter, Boston Scientific, Cork, Ireland), or ENBD or guidewire left in place for several days can be used [60]. Few studies have compared the "stent-by-stent" and "stent-in-stent" bilateral stenting techniques. One retrospectively reviewed the results of these two techniques, but no statistical analysis was performed [61]. There is still no consensus as to which method is more useful to achieve bilateral stenting. However, the "stent-in-stent" method has become more widely used with recent improvements in technique and devices (Table 3) [27,43,57,58,62-66].
CONCLUSIONS
The endoscopic approach has been considered the treatment of choice for palliation of hilar cholangiocarcinoma. Although many endoscopists perform bilateral drainage, a lack of clear consensus on unilateral versus bilateral drainage for hilar cholangiocarcinoma remains. The choice of the number or type of SEMS should be based on the biliary tree configuration, presence of an eventual atrophied lobe, and the need to obtain maximal decompression to eventually offer chemoradiation, and should not be based on operator preference. The decision should also be made based on the availability of expertise at a given institution.
In the near future, spy glass cholangioscope, drug eluting stents, and photodynamic therapy are expected to be used to improve treatment of hilar cholangiocarcinomas.
Acknowledgements
Supported by a 2-year Research Grant of Pusan National University and a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare and Family Affairs, South Korea, No. A091047.
Notes
No potential conflict of interest relevant to this article is reported.