Relationship between bronchial anthracofibrosis and endobronchial tuberculosis
Article information
Abstract
Background/Aims
Various pulmonary diseases may be associated with bronchial anthracofibrosis (BAF). Our aim was to identify a relationship between BAF and endobronchial tuberculosis (EBTB).
Methods
In total, 156 patients, diagnosed with EBTB using bronchoscopy, between June 1999 and May 2008, were included. Clinical and bronchoscopic findings between patients with BAF (n = 72, BAF group) and without BAF (n = 84, non-BAF) were analyzed retrospectively.
Results
The crude odds ratio (OR) of BAF for EBTB was 8.88 (95% confidence interval, 6.37 to 12.37). On multivariate analysis, adjusting for age, history of biomass smoke exposure, and comorbidities, the most significant independent factor for EBTB was a history of biomass smoke exposure (adjusted OR, 17.471; adjusted p < 0.001). EBTB was more frequent in the right lung, particularly the right middle lobar bronchus, in the BAF group. Actively caseating, edematous-hyperemic, and ulcerative were the major types, with 77 (49%), 33 (21%), and 31 cases (20%), respectively. The BAF group had more ulcerative type, while the non-BAF group had more actively caseating type. The duration of EBTB treatment was similar between the groups. No significant difference was observed in the development of complications during treatment and posttreatment bronchostenosis between the groups.
Conclusions
These findings suggest that BAF may be a risk factor for EBTB and affect the location and morphological type at the time of EBTB development.
INTRODUCTION
Endobronchial tuberculosis (EBTB) is known to be a specific inflammatory disease, caused by acid-fast bacilli in the trachea or bronchi and is prevalent in young females [1-4]. However, in Korea, it is also commonly observed in elderly individuals [5,6], particularly in patients with bronchial anthracofibrosis (BAF) [7].
BAF, typically induced by the long-term inhalation of biomass smoke [7], is characterized by a bronchoscopic finding of multiple dark anthracotic pigmentation in the large airway mucosa with or without bronchial narrowing or obliteration [8]. In some patients with EBTB, caused by erosion and perforation of anthracotic lymph nodes into the bronchus from mediastinal tuberculous lymphadenitis, coal pigments may be deposited in the bronchial mucosa [9,10], but this pathological feature is quite different from that observed in BAF [7].
As tuberculosis is one of the most common diseases associated with BAF [7,11], patients with EBTB and BAF seem to have two different pathological processes affecting the same airway mucosa. Consequently, it is expected that their presenting demographic and clinical features would also be different from those in EBTB patients without BAF. The aim of this study was to evaluate any relationship between BAF and EBTB from a comparative analysis according to the presence of BAF.
METHODS
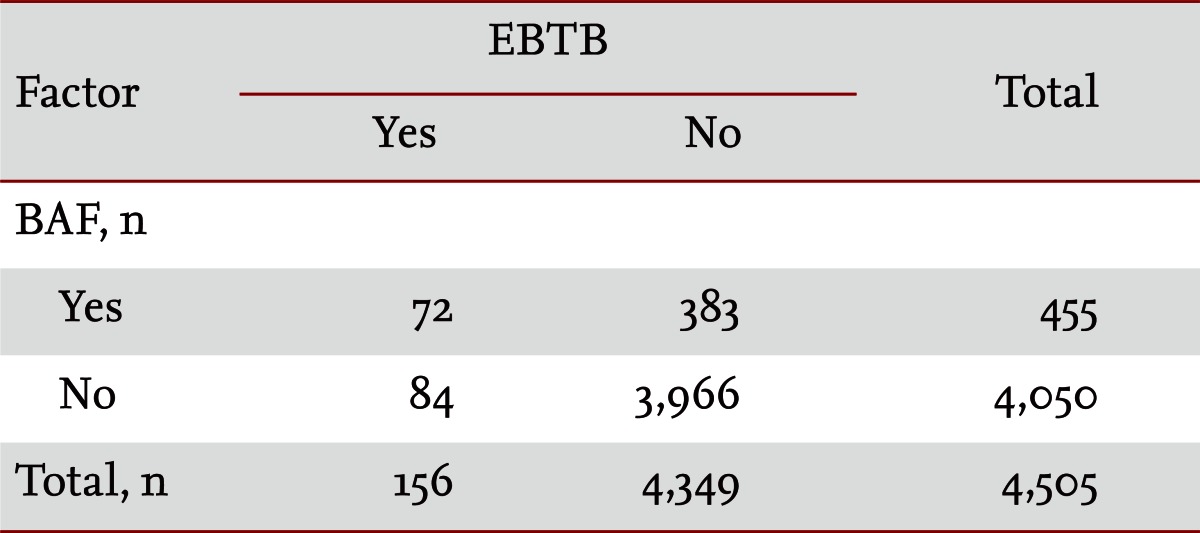
Among the total of 4,505 patients who underwent bronchoscopies between June 1999 and May 2008 at our 700-bed referral hospital, 156 patients found to have EBTB and 455 patients with BAF were included in this study. The clinical features, bronchoscopic findings, and outcomes between the EBTB patients with BAF (n = 72, BAF group) and without BAF (n = 84, non-BAF group) were analyzed retrospectively.
The definition of BAF and history of biomass smoke exposure were described previously [7]. The history of biomass smoke exposure was estimated from the beginning of the patient's time of cooking and heating in a traditional Korean rural housing environment to the time at which changes in the living environment, type of fuel used, or retirement occurred. The diagnostic criteria for active EBTB were: 1) obvious endobronchial lesions observed by bronchoscopy; and 2) tuberculosis, demonstrated by a bronchoscopic biopsy of the lesions or a positive acid-fast bacilli smear from a bronchial washing specimen. The morphological classification of EBTB was based on Chung and Lee [12], as nonspecific bronchitic, granular, hyperemic-edematous, actively caseating, ulcerative, tumorous, and fibrostenotic types. In the case of mixed lesions, we recorded the dominant type. Bronchoscopic findings were based on primary bronchoscopy data, because follow-up bronchoscopy was conducted in only 23 subjects.
All diagnostic procedures were performed with informed consent from patients. The study protocol was approved by the hospital institutional review board.
Data are reported as means ± SD or number (%). The risk factor analysis of BAF for EBTB was measured in terms of the estimated odds ratio (OR) with 95% confidence intervals (CIs). The BAF and non-BAF groups were compared using Student's t test for continuous variables and the chi-squared test for categorical variables. To evaluate independent factors associated with EBTB between the BAF and non-BAF groups, logistic regression was used. The p values of < 0.05 were considered to indicate statistical significance. Data were analyzed using the SPSS software version 14.0 (SPSS Inc., Chicago, IL, USA).
RESULTS
Risk factor analysis of BAF for EBTB
Of the 455 BAF subjects, 72 (15.8%) had EBTB, while among the 4,050 non-BAF subjects, 84 (2.1%) had EBTB. The OR of BAF for EBTB was 8.876 (95% CI, 6.370 to 12.368) (Table 1).
Subjects and clinical characteristics
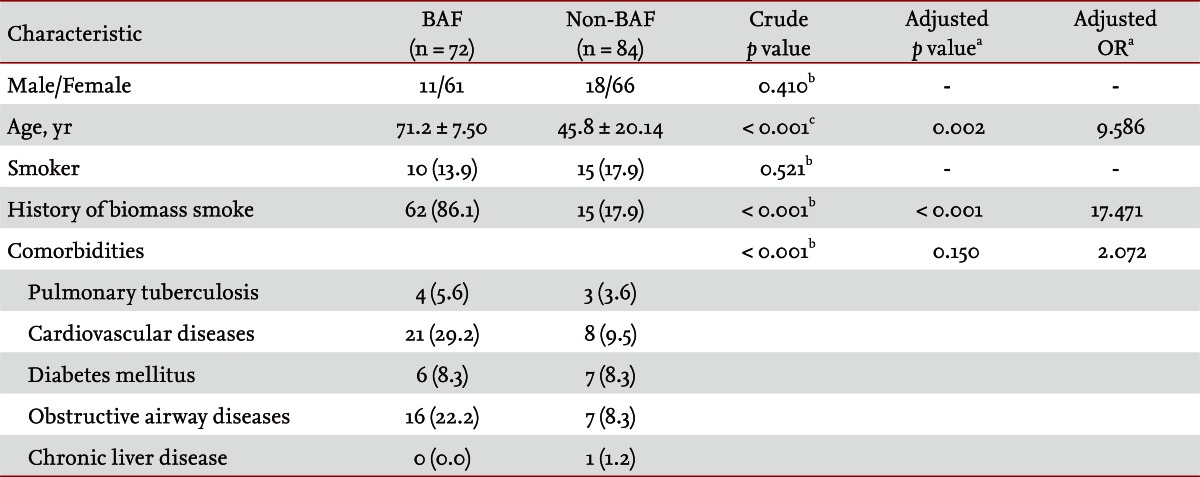
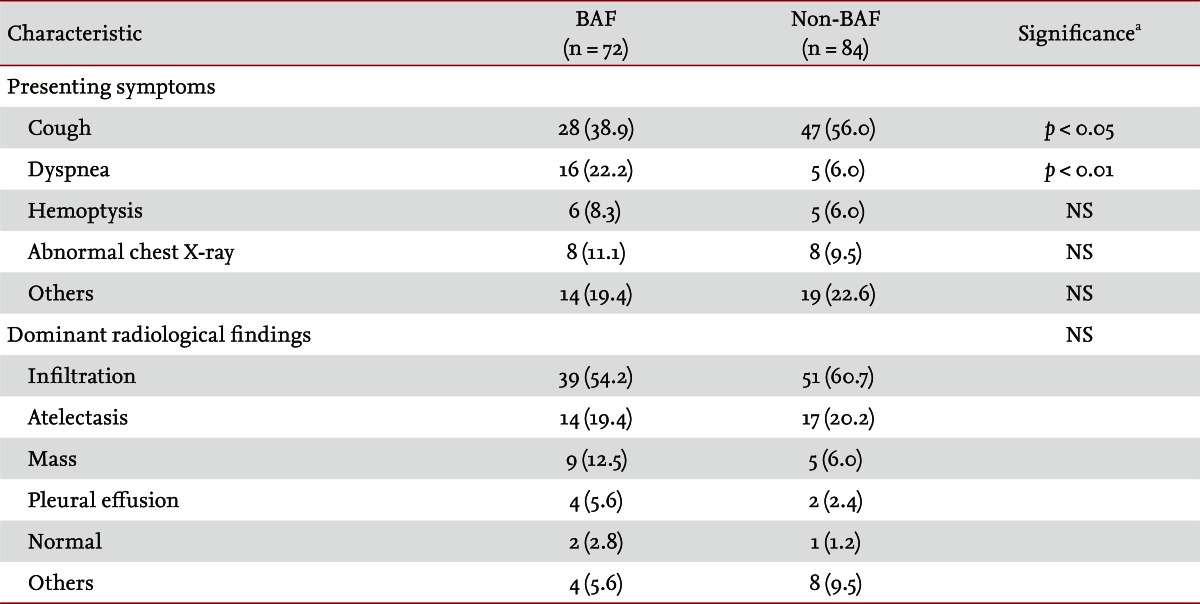
Baseline demographic characteristics of both the BAF and non-BAF groups are summarized in Table 2. Females and nonsmokers were prevalent in both groups, but no significant difference was observed between the groups. The mean age in the BAF group was 71.2 years, significantly older than that of the non-BAF group (45.8 years). The BAF group had a significantly higher frequency of history of biomass smoke exposure and comorbidities. Cardiovascular disease and obstructive airway disease were two most common comorbidities in the BAF group. On multivariate analysis, adjusted for age, history of biomass smoke, and comorbidities, the most significant variable was a history of biomass smoke exposure (adjusted OR, 17.471; adjusted p < 0.001). Cough was the most common presenting symptom and was significantly more frequent in the non-BAF group, while dyspnea was more frequent in the BAF group. Infiltration and atelectasis were common dominant radiological findings, followed by mass and pleural effusion, but no significant difference was noted between the groups (Table 3).
Bronchoscopic findings
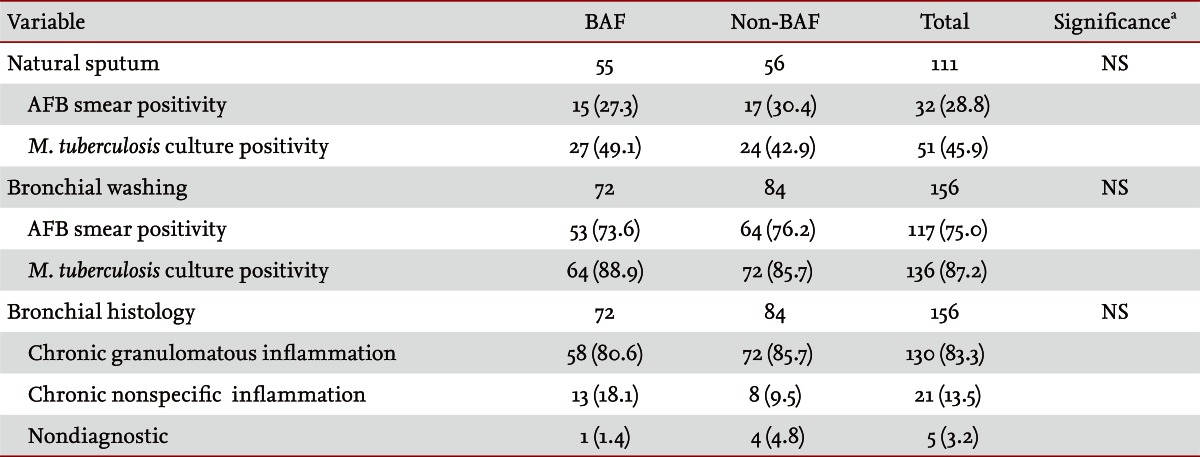
The right lung was a commonly involved site of EBTB in 97 patients (62%), particularly the right middle lobar bronchus in 39 patients (25%) and the right upper lobar bronchus in 32 patients (21%). In the left lung, the left upper lobar bronchus was a common site of EBTB, in 28 patients (18%). EBTB was observed significantly more commonly in the right lung, especially in the right middle lobar bronchus, in the BAF group. However, EBTB was more significantly observed in the left lower lobar bronchus in the non-BAF group than in the BAF group. By morphological classification of EBTB, actively caseating, edematous-hyperemic, and ulcerative types were the predominant types observed, in 77 (49%), 33 (21%), and 31 cases (20%), respectively (Fig. 1). Patients with BAF had more ulcerative type, while patients without BAF had more actively caseating type disease (Table 4). The rates of AFB smear and Mycobacterium tuberculosis culture positivity frequencies in sputum were 28.8% and 45.9%, respectively, and in bronchial washings, 75% and 87.2%, respectively. The most frequently observed histological feature was chronic granulomatous inflammation (83.3%), followed by chronic nonspecific inflammation (13.5%) and nondiagnostic (3.2%). No significant difference in diagnostic yield was observed in any sample between the groups (Table 5).
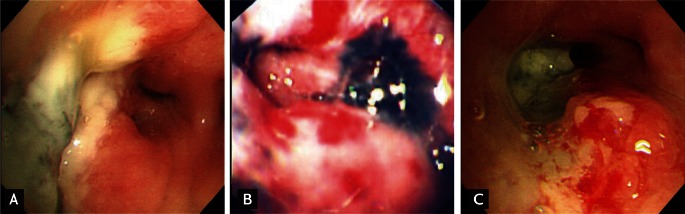
Three common types of endobronchial tuberculosis in patients with bronchial anthracofibrosis. (A) Actively caseating type in the right middle lobar bronchus. (B) Hyperemic-edematous type in the left upper lobar bronchus. (C) Ulcerative type in the right middle lobar bronchus, associated with a hyperemic-edematous surrounding mucosa.
Outcomes
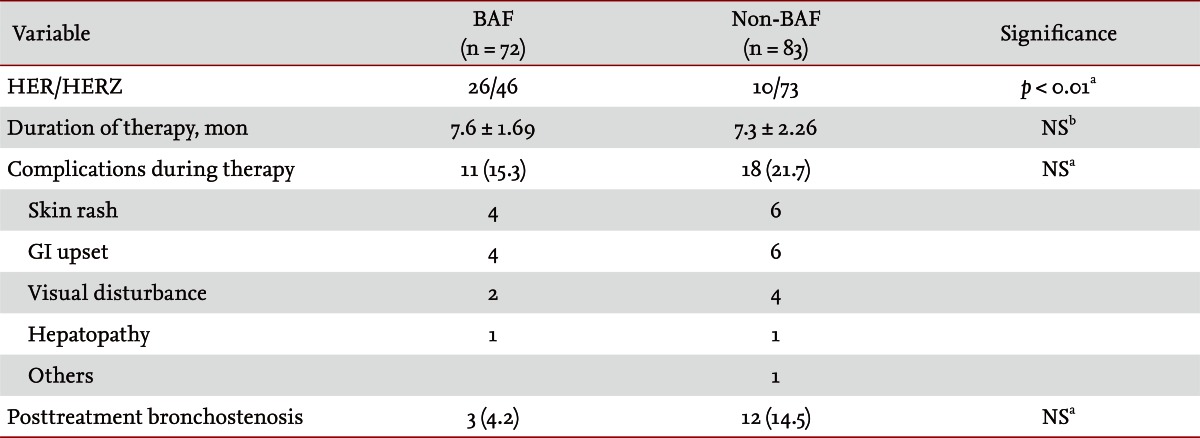
Among the non-BAF group, one patient was transferred to a primary hospital. For treatment of EBTB, most patients started with the isoniazid (H), ethambutol (E), rifampicin (R), and pyrazinamide (Z) regimen. In the non-BAF group, the proportion of patients treated with the HERZ regimen was significantly higher than that of the BAF group. The duration of treatment was 7.6 months in the BAF group and 7.3 months in the non-BAF group. No significant difference was seen in development of complications during treatment or posttreatment bronchostenosis between the two groups (Table 6).
DISCUSSION
In this study, patients who had BAF on bronchoscopic examination had a higher chance of EBTB than those without BAF (OR, 8.88). Among the EBTB patients, the BAF group was significantly older than the non-BAF group and had a significantly higher frequency of history of biomass smoke exposure and comorbidities. On multivariate analysis, history of biomass smoke exposure was the most important factor for EBTB. Previous studies have reported high rates of tuberculosis in patients with BAF [7,8,13]. Most BAF patients in Korea were older females and had experienced long-term exposure to smoke from biomass fuels, such as wood, leaves, and crop residues [7]. Thus, the reasons for the high rates of tuberculosis in patients with BAF may be as follows: first, an association with biomass smoke as an independent risk factor for pulmonary tuberculosis [14-17]; second, an increase in tuberculosis prevalence related to old age [18]; and third, compromise of pulmonary and bronchial immune defense mechanisms due to exposure to toxic substances in biomass smoke or the pathology of BAF itself [7,19]. The results of the present study are consistent with this explanation.
EBTB shows diverse symptoms, such as chronic cough, hemoptysis, and dyspnea accompanied by sputum, that are not alleviated readily by conservative treatments. In our series, cough and dyspnea were the most common symptoms. In particular, dyspnea was seen frequently in the BAF group, as this group consisted mostly of elderly patients who had significantly higher frequencies of comorbidities, particularly obstructive airway diseases and cardiovascular diseases.
In chest X-ray findings of EBTB, as in this study, infiltration, consolidation, and atelectasis predominate; however, normal findings have been reported in 3% to 8.3% of cases [1,2,4-6]. BAF radiological examinations show primary and secondary findings [7]. The characteristic primary finding is curvilinear bronchial narrowing or atelectasis with enlargement of lymph nodes, which are usually calcified, and are seen commonly with chest computed tomography [8,20]. However, secondary findings are determined by associated diseases [7,8,20]. Although atelectasis or calcified lymph nodes are encountered occasionally, most simple chest radiographic findings are secondary, such as consolidation, interstitial infiltration, and pleural lesions [7]. From this point of view, in our series, no significant difference between the two groups in dominant radiological findings with simple chest X-rays would be expected. Thus, these two diseases are difficult to discriminate using simple chest radiographic findings. Generally, bronchoscopic examination is not only useful for diagnosing EBTB, but also for evaluating the prognosis by observing the degree of progression and morphology. It is also an essential test in diagnosing BAF.
The pathophysiological mechanisms of EBTB are thought to involve direct invasion onto a bronchus from an infected lung parenchyma, implantation from infected sputum, hematogenous spread, erosion into a bronchus from a tuberculous lymph node, or extension into the peribronchial region by lymphatic drainage from an infected parenchyma [21]. In the present study, EBTB was more frequent in the right lung (62.2%), and the upper lobar (38.5%) and right middle lobar bronchi (25%) were the most frequent sites. This trend was even more prevalent in the BAF group. These findings are likely because these regions include not only the most common site of pulmonary tuberculosis [2,5,22] but also the main pathological site of BAF [7,23]. The principal pathological bronchial finding of BAF was multiple pigmentation and bronchial stenosis [7,8]. In particular, the right middle lobar bronchus is the most common site of bronchial stenosis, due to its anatomical location [7]. This pathology may alter the local immune defense mechanism and provide a good place for the implantation of M. tuberculosis from any origin [7,19].
Morphological classification of EBTB was conducted according to Chung and Lee's method [12], as nonspecific bronchitic, granular, hyperemic-edematous, actively caseating, ulcerative, tumorous, and fibrostenotic types. This classification scheme provided us with not only the morphological types but also the degree of disease progression relatively well. Moreover, the therapeutic outcome of each type, except the tumorous type, can be predicted by follow-up bronchoscopy during the initial 2 to 3 months of treatment [12]. As in previous studies [4-6,12], in our series, actively caseating and edematous-hyperemic types were encountered most frequently, in 49.4% and 21.8% of cases, respectively. Various bronchial findings can evolve within 3 months after clinical presentation [12,24]. Differences in the times for bronchoscopic diagnoses of EBTB may be partially responsible for the somewhat different composition of morphological types between the two groups. The ulcerative type was also frequently observed (19.9%), particularly in the BAF group. This may be explained by the fact that EBTB is accompanied locally by regions of severe anthracofibrotic change, and tissue destruction develops rapidly (Fig. 1C) [7]. Because mucus entrapment by proximal bronchial granulation tissue in EBTB can prevent expectorating sputum, as in our cases, the diagnostic yield on sputum AFB smears was generally poor in studies with large numbers of cases [2,4,12]. Accordingly, bronchoscopic sampling has been key to diagnosis. However, the presence of BAF could not alter the diagnostic yield on any sample for EBTB.
For EBTB treatment, we used either the HERZ or HER regimen for 6 to 9 months or longer, as needed. The HER regimen was used more in the BAF group, because we had several older patients, compared with the non-BAF group. However, no significant difference in the development of complications during treatment and posttreatment bronchostenosis was observed between the two groups. Controversy regarding the effects of corticosteroids in EBTB remains [25-29], but we use these in a limited fashion in cases with tracheal or main bronchial involvement or in cases with actively caseating type in the upper lobar bronchus with invasion to the proximal portion of the main bronchus.
According to Chung and Lee [12], all subtypes of EBTB can transform into another subtype during treatment, and bronchial stenosis is inevitable if the disease progresses beyond a critical point, determined mainly by the extent of disease progression and related closely to granulation tissue formation. In the present study, in just 15 cases with clinically suspected bronchostenosis after treatment, we performed a follow-up bronchoscopy and observed mild bronchial stenosis, which did not need an interventional procedure. Although we could not monitor changes in morphology or the degree of stenosis in all subjects, it seems that the presence of BAF did not alter the natural course of EBTB or treatment outcomes.
In conclusion, BAF is an emerging pulmonary disease worldwide due to biomass fuel exposure [30]; indeed, it is estimated that over 50% of all households and 90% of all rural households worldwide continue to use biomass fuel as their main domestic source of energy [31]. The structural, physiological, and local immunological abnormalities of the disease may lead to the development of multiple pulmonary diseases [7,30]. Our data suggest that BAF is a risk factor for EBTB and affects the location and morphological type at the time of EBTB development. Because this study was limited by its retrospective design, further prospective studies are needed to clarify the relationship between the presence of BAF and the natural course and treatment outcome of EBTB.
KEY MESSAGE
1. Patients who had bronchial anthracofibrosis (BAF) had a higher chance of endobronchial tuberculosis (EBTB) than those without BAF.
2. EBTB was more frequent in the right lung, particularly the right middle lobar bronchus, and was more ulcerative in the BAF patients.
3. BAF can be a risk factor for EBTB and affect the location and morphological type of EBTB.
Notes
No potential conflict of interest relevant to this article is reported.