Clinical characteristics and outcomes in renal transplant recipients with renal cell carcinoma in the native kidney
Article information
Abstract
Background/Aims
We investigated the incidence and clinical characteristics of renal cell carcinoma (RCC) in the native kidney of renal transplant recipients.
Methods
Between 1991 and 2010, 1,425 patients underwent kidney transplantation at our institution. We retrospectively evaluated the clinical features and outcomes in renal transplant patients with RCC in the native kidney after renal transplantation.
Results
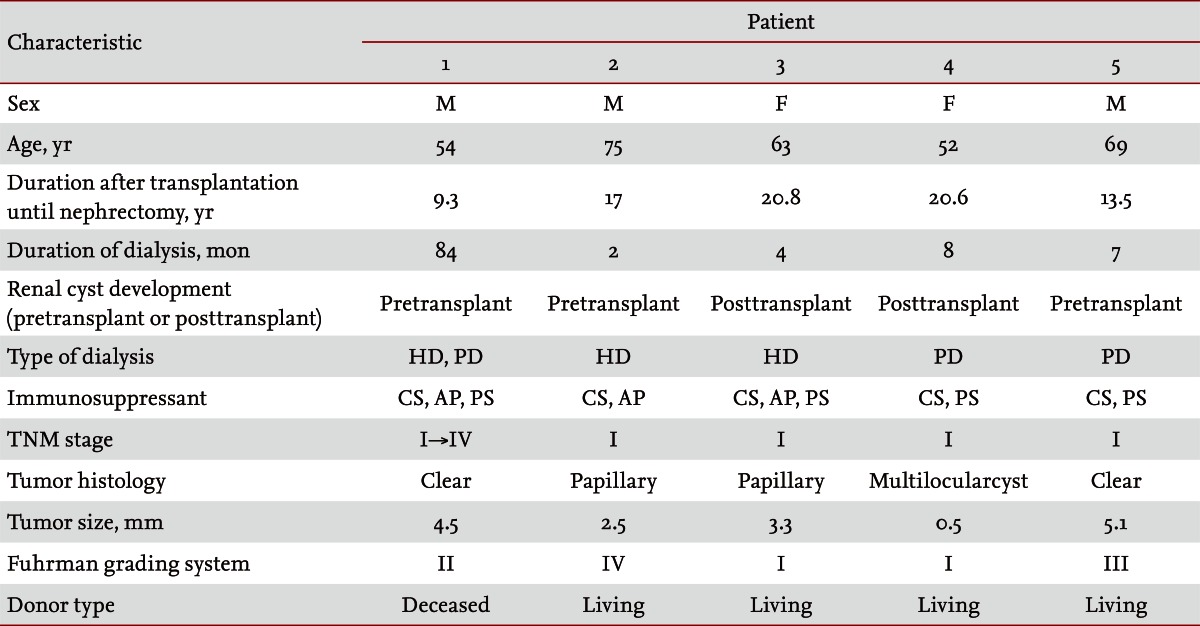
The patients included three males and two females with a mean age of 63 years (range, 52 to 74). The incidence of RCC was 0.35%. The median interval between renal transplantation and RCC occurrence was 16.2 years (range, 9 to 20). All of our patients with RCC had developed renal cysts either before (n = 3) or after (n = 2) renal transplantation. The mean duration of dialysis was 12 months (range, 2 to 39). Of the five patients, four underwent dialysis treatment for less than 8 months. All the RCCs were low grade at the time of diagnosis. Four patients underwent radical nephrectomy, and one patient refused the operation. The four patients who underwent radical nephrectomy showed no evidence of local recurrence or distant metastasis during the median follow-up of 2.9 years. However, the patient who did not undergo surgery developed spinal metastasis from the RCC 6 years later.
Conclusions
This study suggests that the follow-up period is an important factor for the development of RCC in renal transplant recipients, and more vigorous screening with a longer follow-up period is required in renal transplant recipients.
INTRODUCTION
With longer graft survival in transplant recipients and the introduction of more potent immunosuppressive medications, malignancies contribute to mortality in 9% to 16% of renal transplant recipients [1]. The overall incidence of malignancy after renal transplantation is three to five times higher than that in the general population [2,3], and urinary tract cancer is reported as the third most common malignancy after renal transplantation [4].
The incidence of renal cell carcinoma (RCC) is reported to be 15 times higher in the native kidney of patients after renal transplantation than in the general population [5]. Renal cysts and long dialysis duration are risk factors for RCC in renal transplant recipients [6]. The probability of RCC among patients with acquired cystic kidney disease (ACKD) is 4% to 7% over a 7- to 10-year period [7,8], with an annual incidence of 0.18% [9]. Whereas most RCCs are low-grade tumors with favorable prognoses [10], the prognosis of metastatic RCC is very poor [11].
In this study, we investigated the incidence and clinical characteristics of RCC in the native kidney of renal transplant recipients at a single healthcare center.
METHODS
Between 1991 and 2010, 1,425 patients underwent kidney transplantation at our institution. Of these, five (0.35%) developed malignancy in the native kidneys. We retrospectively evaluated the clinical features and outcomes of the native kidneys in these five patients with RCC in the native kidney after they underwent renal transplantation. This study was approved by the Institutional Review Board of Seoul St. Mary's Hospital.
We investigated the incidence of RCC in the native kidneys of renal transplant recipients, the duration of dialysis before transplantation, the time period from the onset of RCC to the time of transplantation, the use of immunosuppressives, and the prognosis. Radical nephrectomy was performed when computed tomography confirmed the presence of an irregular cyst wall or a solid tumor. The presence of a renal cyst, the size, stage and Fuhrman grade of the surgical specimens were recorded.
RESULTS
The baseline characteristics of the patients with RCC are presented in Table 1. The patients included three males and two females with a mean age of 63 years (range, 52 to 74). The mean follow-up period after renal transplantation was 16.2 years (range, 9 to 20). The patients had developed renal cysts before (n = 3) or after (n = 2) renal transplantation. The mean duration of dialysis was 12 months (range, 2 to 39), and all patients except one underwent dialysis treatment for less than 8 months. Four patients who underwent dialysis for less than 8 months received living donor allografts. Four patients were asymptomatic, and one complained of vague abdominal discomfort. The asymptomatic patients were diagnosed using routine ultrasonography. All patients received standard immunosuppressive treatment at the time of RCC diagnosis. Of the five patients, three received dual therapy with cyclosporine and prednisolone, and two patients received triple therapy with cyclosporine, azathioprine, and prednisolone.
After RCC diagnosis, four patients underwent radical nephrectomy, and one patient refused the operation. The four patients who underwent the surgery showed no evidence of local recurrence or distant metastasis during the follow-up period (median follow-up duration, 2.9 years). The patient with RCC who did not undergo surgery developed spinal metastasis 6 years later; he died despite undergoing radical nephrectomy at that time. Analysis of the surgical specimens showed that the mean tumor size was 3.2 cm (range, 0.5 to 5.1) and that all the RCCs were low grade at the time of diagnosis. Two patients had clear cell carcinoma; two patients had papillary RCC; and one patient had multilocular cystic RCC. The tumors were graded according to the Fuhrman grading system: two tumors were grade I, one was grade II, one was grade III, and one was grade IV.
DISCUSSION
In a follow-up period of an average of 16.2 years, the cumulative incidence of RCC after transplant in five renal transplant recipients with was 0.35%, which translates to 20 RCCs in 100,000 renal transplant recipient years. According to data from the Korean Central Cancer Registry in 2007, the incidence of RCC in the general population was 5.8 of 100,000 persons [12]. Thus, the incidence of RCC in this study was 3.4-time that in the general population, which was relatively low considering that RCC has been reported to be approximately 15 times more common in renal transplant recipients than in the general population of the United States [5]. Stafford et al. [13] reported that such racial disparity in RCC incidence might be due to biological, behavioral, and environmental factors.
Renal cysts and ACKD are major risk factors for RCC in renal transplant recipients [6,14]. Nephron loss associated with renal insufficiency may promote hyperplasia of tubular epithelial cells, resulting in cyst formation, and atypical epithelial proliferation in the cyst may represent a precursor lesion to RCC [15]. In this study, all patients had renal cysts at the time they were diagnosed with RCC. Three of the five patients developed renal cysts before renal transplantation and the remaining two patients developed renal cysts after transplantation. Ishikawa et al. [16] suggested that renal cyst formation may cease or regress following renal transplantation with removal of the uremic milieu. However, in our study, two patients (40%) developed renal cysts after renal transplantation; this result is similar to previous reports that renal cyst development is not arrested after transplantation [6,14]. Therefore, it is important to screen the native kidney in renal transplant recipients after renal transplantation, regardless of the presence of a renal cyst.
Long duration of dialysis is associated with ACKD and is reported as a risk factor for RCC in renal transplant recipients [6,14,17]. In a recent study, dialysis treatment for more than 4.6 years was found to be associated with RCC in renal transplant recipients [14]. However, in our study, the mean duration of dialysis was 12 months (range, 2 to 39), and four patients underwent dialysis treatment for less than 8 months. These four patients received living donor allografts within 8 months of dialysis initiation. This transplantation explains the shorter duration of dialysis treatment in our cases compared to those in previous studies. Thus, it is necessary to consider the possibility of RCC in the native kidney of patients with dialysis treatment of short duration.
The median interval between renal transplantation and RCC occurrence was 16.2 years (range, 9 to 20), which is relatively long considering that the interval ranged from 4.5 to 10.5 years in previous reports [6,10,11,17]. A greater number of cancers may develop and be detected if patients are followed up for longer time. Hwang et al. [18] reported that a longer follow-up period after kidney transplantation is associated with cancer in renal transplant recipients. Thus, vigorous cancer screening is required in renal transplant recipients with longer follow-up periods. For early RCC detection, Goh and Vathsala [6] recommended that regular ultrasonography be performed within 1 month of transplantation, every 5 years thereafter for those without cysts, and every 2 years thereafter for those with cysts. However, no guidelines exist for RCC screening in recipients after renal transplantation.
In general, it has been reported that the prognosis of low stage RCC in the native kidney of renal transplant recipients is favorable [10]. In contrast, the prognosis of metastatic RCC is very poor [11]. All RCCs in our study were low grade at the time of diagnosis. After RCC detection, radical nephrectomy was performed in four of five patients; one patient refused to undergo the operation. The four patients who underwent radical nephrectomy showed no evidence of local recurrence or distant metastasis during the median follow-up of 2.9 years. However, the patient who refused to undergo the operation presented with spinal metastasis of RCC 6 years later and died despite undergoing radical nephrectomy. Therefore, nephrectomy should be performed immediately when RCC is diagnosed in renal transplant recipients.
In summary, the incidence of RCC was 0.35% in this study. Renal cysts, a major risk factor for RCC in renal transplant recipients, were detected in all subjects. The mean follow-up period in this study was 16.2 years, which was longer than reported previously. Our findings suggest that the follow-up period is an important factor for development of RCC in renal transplant recipients, and more vigorous cancer screening with a longer follow-up period is needed in renal transplant recipients.
KEY MESSAGE
1. The risk for renal cell carcinoma (RCC) in native kidneys after renal transplantation is substantial.
2. Follow-up period is an important factor for development of RCC in renal transplant recipients.
3. Therefore, more aggressive cancer screening is needed in renal transplant recipients with a longer follow-up period.
Acknowledgments
This study was supported by a grant (A102065) from the Korea Healthcare Technology R&D Project, Ministry of Health and Welfare, Republic of Korea.
Notes
No potential conflict of interest relevant to this article is reported.