Effects of D-002, a mixture of high molecular weight beeswax alcohols, on patients with nonalcoholic fatty liver disease
Article information
Abstract
Background/Aims
Nonalcoholic fatty liver disease (NAFLD) is intimately related to insulin resistance and ranges from a benign course to liver fibrosis and cirrhosis. NAFLD management mainly involves dietary modification and weight loss. Although no fully successful pharmacological intervention is available, alternative therapies to treat NAFLD have shown promising results. Experimental studies have shown that D-002, a mixture of beeswax alcohols with antioxidant effects, is hepatoprotective. The aim of this study was to investigate the efficacy and safety of D-002 in patients with NALFD.
Methods
Fifty patients with NAFLD were randomized to receive a placebo or D-002 (100 mg/day) for 24 weeks. The primary endpoint was a significant ultrasonography-detected reduction of liver fat infiltration versus a placebo. Secondary endpoints were decreases in the homeostatic model assessment (HOMA) index, insulin levels, serum liver enzymes, increases in plasma total antioxidant status (TAS) and improved clinical symptoms versus the placebo recipients.
Results
At randomization, all indicators were comparable in both groups. At study completion, seven (28.0%) D-002-patients, but none of the placebo recipients, exhibited a normal liver echo pattern on ultrasonography (p < 0.01). Also, D-002 significantly reduced (p < 0.01 vs. baseline and placebo) the HOMA index and insulin levels and increased the TAS, but did not affect other parameters. The proportion of D-002-patients (12/25, 48.0%) showing symptom improvement was higher (p < 0.001) than that of the placebo group (1/25, 4.0%). The treatment was safe and well tolerated. Three patients in each group withdrew from the study.
Conclusions
D-002 (100 mg/day) improved ultrasonographic findings, indicators of insulin resistance, plasma TAS and clinical evolution on NAFLD patients. Further studies, however, are needed to confirm these results.
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD), a common liver condition, is characterized by excessive accumulation of hepatic fat in the absence of significant alcohol consumption, and has been associated with insulin resistance (IR) [1-4]. Histologically, NAFLD accounts for the fat accumulation, mainly triglycerides (TG), into the hepatocyte cytoplasm (5% to 10% of the organ weight) [4,5].
The prevalence of NAFLD ranges from 10% to about 83%, depending on the population and research criteria, and it is overrepresented in diabetics, the obese, and females [1-4], although it may be also present in nonobese and nondiabetic subjects [6]. NAFLD generally follows a benign course and remains stable for years, but many undiagnosed cases may evolve to nonalcoholic steatohepatitis (NASH), liver fibrosis and cryptogenic liver cirrhosis [1-4].
NAFLD management involves dietary modification and weight loss [6-9]. Although some treatments (insulin sensitizers, antioxidants, lipid-lowering drugs, and ursodeoxycholic acid, among others), have proven beneficial in patients with NFALD, no fully successful pharmacological intervention is available [1,6-12].
In the search for effective and safe alternative therapies, the effects of some natural products on NAFLD have been investigated. The use of n-3 polyunsaturated fatty acids (PUFA) (2 g/day) from seal oils has proven to safely and effectively improve symptoms and normalize ultrasonographic features, as well as improve serum alanine aminotransferase (ALT) and lipid levels [13].
Also, p olicosanol, a mixture of high molecular weight alcohols isolated from sugarcane wax, has effectively treated IR in hyperlipidemic patients with fatty liver disease, significantly lowering the homeostatic model assessment (HOMA) index and total cholesterol (TC) and low density lipoprotein cholesterol (LDL) levels [14], consistent with the fact that octacosanol, its main component, attenuates the disrupted metabolism of hepatic reactive oxygen species linked with acute liver injury in carbon tetrachloride (CCl4)-intoxicated rats [15].
D-002, a substance purified from beeswax, contains a mixture of six of the higher aliphatic alcohols (C26, C26, C28, C30, C32, and C34) present in policosanol [16,17], but in different proportions, so that the major component of D-002 is triacontanol (C30), followed by octacosanol (C28) and docotriacontanol (C32). Oral administration of D-002 has been shown to produce antioxidant effects in experimental [18-20] and clinical studies [21-23], and to exhibit hepatoprotective effects in experimental models of CCl4-induced liver injury [24].
The effects of D-002 treatment in patients with NALFD, however, had not been previously investigated. This study was therefore performed to investigate the efficacy and safety of D-002 in patients with NALFD.
METHODS
Study design
This study was a prospective, single-center, randomized, double-blind, placebo-controlled trial consisting of a screening visit and a 24-week treatment period.
The study protocol was approved by the Ethics Committee of the Medical Surgical Research Centre (Havana, Cuba), a Good Clinical Practices-accredited institution. The study was conducted in accordance with the principles of the Declaration of Helsinki. Patients were provided oral and written explanations of the nature of the trial and the treatment before they provided informed written consent (visit 1).
Eligible patients (visit 2) were randomized to D-002 (50 mg) or placebo tablets to be taken twice daily for 24 weeks, and were advised to follow a low-fat low-energy diet (50% carbohydrates, 20% protein, 30% fat) [25]. These dietary conditions were supposed to be followed from enrollment to the end of the study.
Subjects were seen at weeks 6, 12, 18, and 24 weeks (visits 3 to 6) of the treatment period. Physical examinations (determination of body mass index [BMI], blood pressure, and heart rate) and clinical assessments were conducted at each visit. Treatment compliance and adverse events (AEs) were controlled from visits 3 to 6, laboratory testing was performed at baseline and every 12 weeks, while hepatic fat infiltration was assessed by upper abdominal ultrasonography at baseline and week 24.
Patients
We enrolled patients of both sexes, aged 25 to 70 years, with a prior diagnosis of NAFLD and/or persistent increase in liver enzymes without excessive alcohol ingestion (weekly consumption ≥ 70 g in female, ≥ 40 g in male), confirmed by careful questioning of the patients and their primary doctors.
Enrolled patients were eligible for randomization if liver fat infiltration was confirmed by ultrasonography [26].
Exclusion criteria included overuse of alcohol, viral hepatitis, hemochromatosis, Wilson disease, autoimmune hepatitis, primary sclerosing cholangitis, or primary biliary cirrhosis; history of any other hepatic disorder, glucose values > 7 mmol/L (uncontrolled diabetes), inability to provide written informed consent; pregnancy, breastfeeding, and lack of effective birth control in women of child-bearing age. Also, patients who had had unstable angina, myocardial infarction, stroke or any serious AE within the 3 months prior to the study were excluded.
Moreover, patients were excluded if they were receiving any treatment that could influence liver function.
Treatment
The study drugs (D-002 or identical placebo tablets) were taken twice per day (at lunch and dinner) for 24 weeks, so that treated patients received D-002 100 mg/day, a dose within the range approved in humans [27].
Randomization was computer-generated using blocks and a 1/1 randomization ratio. Treatments were given in identical coded packages accordingly. Consumption of drugs with recognized or suggested antioxidant, liver protective, or hepatotoxic effects was not allowed.
Treatment compliance was assessed by counting the remaining tablets with respect to those that should be consumed in each period. To be acceptable, ≥ 85% of the tablets scheduled for a period must have been consumed. Diet adhesion was followed by using special chart records filled by patients and reviewed by doctors, and by recording body weight at each visit.
Efficacy variables
The primary endpoint was a significant reduction in liver fat infiltration as assessed by ultrasonography, as compared to the placebo recipients.
Secondary outcomes
The secondary endpoints were decreases in the HOMA index, insulin levels, and serum liver enzymes, increase in plasma total antioxidant status (TAS), and improved clinical symptoms as compared to the placebo.
Efficacy measurements
Ultrasonographic assessment
All patients underwent ultrasonography for liver steatosis after fasting for 10 to 12 hours. Ultrasound scans were performed by a trained operator who was blind to participants' group allocations.
Steatosis severity was scored using the following scale: 0 (normal echogenicity), 1 (mild), 2 (moderate), 3 (severe) [28]. Liver fat infiltration was considered mild when increased echogenicity plus augmented liver size was present, moderate if these two features were present plus sound attenuation, and severe if the walls of the portal vessels and the diaphragm were unseen.
Clinical evaluation
Patients were questioned about the evolution of their symptoms, such as abdominal discomfort or pain, abdominal bloating, and/or nausea. The changes were classified as follows: improved, worsened or unchanged.
Safety and tolerability
Data from the physical examination (determination of bodyweight, BMI, pulse rate, and arterial pressure), laboratory tests and requests for AE were used for the safety and tolerability analysis. AE was predefined as mild when it did not require the withdrawal of study medication, moderate when it required therapy discontinuation according to physicians' criteria and/or specific treatment of the AE, and serious when it led to or prolonged hospitalization, which could be fatal or nonfatal.
Laboratory analysis
Blood samples were drawn from 8:00 to 8:30 AM. After an overnight fasting of 12 hours, and aliquots were obtained for laboratory determinations.
Liver enzymes (ALT, aspartate aminotransferase [AST], γ glutamyl transferase [GGT], alkaline phosphatase [ALP]), serum glucose, creatinine, bilirubin, TC, high density lipoprotein cholesterol (HDL-C), and TG were determined by enzymatic methods using reagent kits (Roche, Basel, Switzerland). LDL-C values were calculated using the Friedewald equation [29]. Insulin levels were determined using radioimmunoassay and the prothrombin time with the routine laboratory method.
The HOMA index was calculated as follows: HOMA = [fasting glucose (in mg/dL) / 18] × [fasting insulin (in µUI/mL) / 22.5]. Plasma TAS was assessed by using a reagent kit from Randox (NX2332, Randox, Antrim, UK) and values were reported in mmol/L.
Statistical analyses
Analyses were conducted in accordance with the intention to treat principle. The data of all randomized patients were analyzed. Sample size was calculated by assuming that the mean reduction from baseline of liver fat infiltration should be greater than 25% with respect to the change in the placebo recipients. A sample size of 25 subjects per group was estimated to be sufficient to detect such differences at a 5% level of significance with 80% power.
The data are presented as means ± SD. Continuous variables were analyzed using the Wilcoxon test for matched samples (within-group comparisons) and Mann-Whitney U test (between-group comparisons), being confirmed using tests for paired and independent samples, respectively. Categorical values were compared using Fisher's exact probability test and confirmed with the Ψ2 test. Two-side tests were used. A value of p < 0.05 was considered to indicate statistical significance.
RESULTS
Characteristics of the study patients
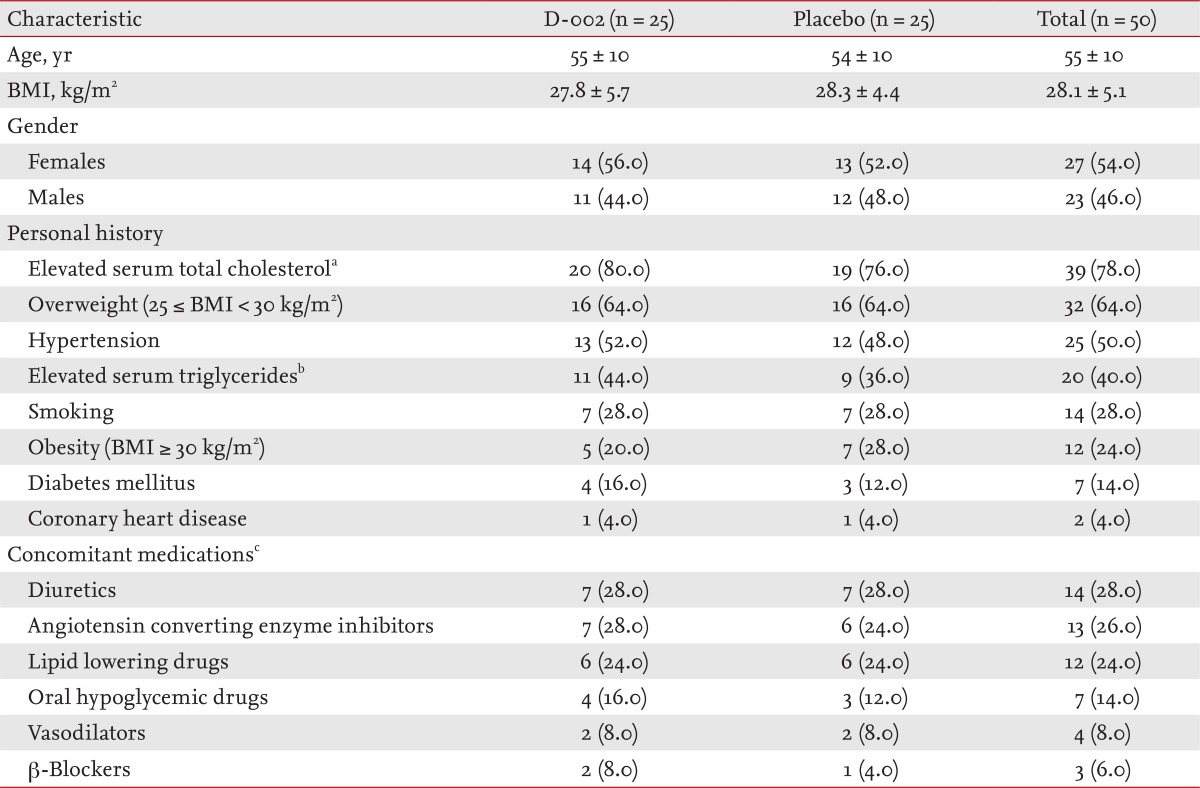
Of the 55 patients enrolled in this study, 50 were randomized to D-002 (25) or placebo (25). Five subjects were not included because they had no ultrasonographic evidence of fat infiltration. Six patients, three in each group, withdrew from the study; two of the placebo recipients withdrew due to AE.
Table 1 shows the baseline characteristics of the patients. Patients (27 females, 23 males; mean age, 55 years) in both groups were matched for all characteristics. Increased body mass (64.0%), elevated serum TC (78.0%), hypertension (50.0%), elevated serum TG (40.0%), smoking (28.0), and obesity (24.0%) were the most frequent (≥ 20%) findings in the personal history of the patients. Twenty-four of the 50 patients had concomitant hypertension, serum TG ≥ 1.7 mmol/L, HDL-C < 1.00 mmol/L, and fasting glucose ≥ 5.6 mmol/L.
Concomitant medications, also comparable in both groups, were consistent with the personal history of the participants; antihypertensive, lipid-lowering, and oral hypoglycemic drugs were among the concomitant therapy taken most commonly by the study participants.
Efficacy analysis
No significant difference between the groups in dietary and treatment compliance was observed.
Effects on the primary efficacy outcome
Fig. 1 summarizes the ultrasonographic findings. At baseline, the stages of steatosis were comparable in both groups; no patient was classified as normal (score 0). In addition, 20, 24, and six patients had mild, moderate and severe steatosis, respectively, all degrees of liver fat infiltration being balanced in both groups. Seven (28.0%) D-002-patients and none of the placebo patients exhibited a normal liver echo pattern on ultrasonography at study completion (p < 0.01). Only 3/25 (12%) patients, all in the placebo group, demonstrated severe steatosis at the end of the study.
Effects on secondary efficacy outcomes
IR and other blood efficacy outcomes
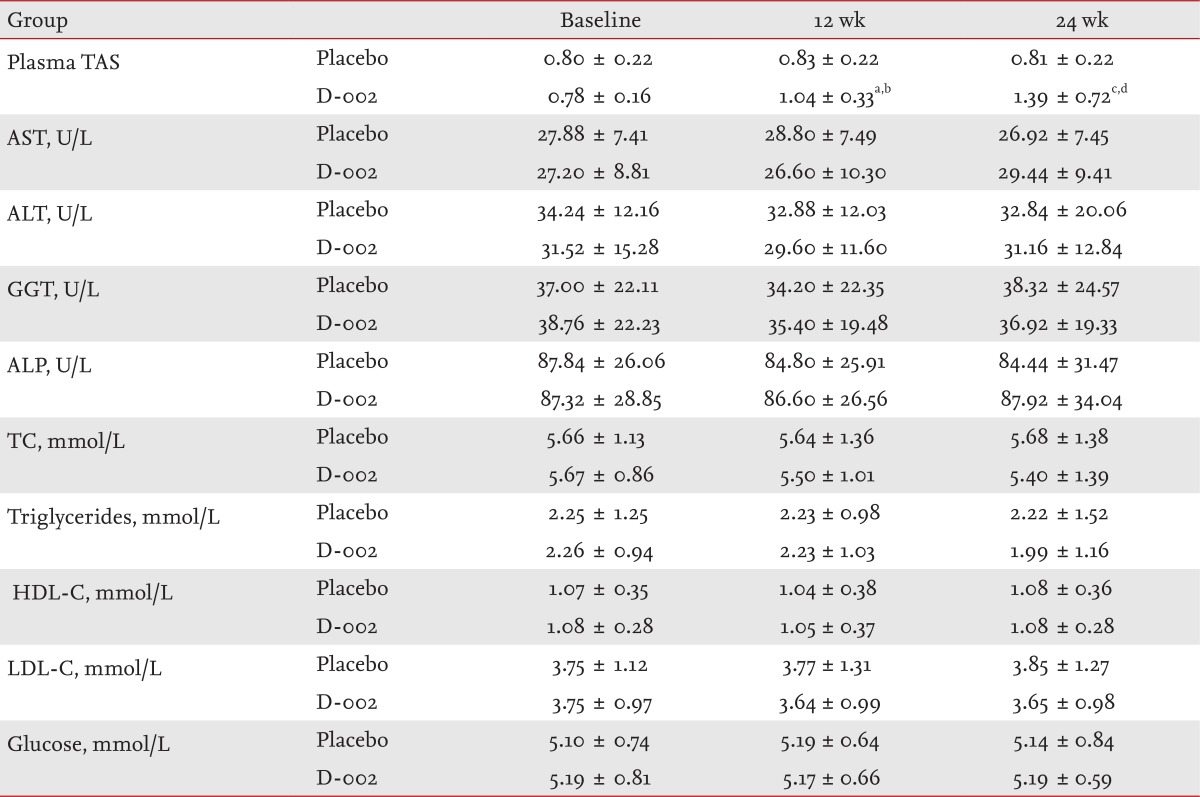
D-002 treatment significantly reduced insulin levels and the HOMA index by 37% and 39.9%, respectively (p < 0.01 as compared to baseline and placebo) (Fig. 2); meanwhile, the TAS increased significantly, while the liver enzymes, serum glucose and lipid profile remained constant (p < 0.0001 as compared to baseline and placebo) (Table 2).

Secondary efficacy outcomes related to insulin resistance. (A) Insulin levels (µUI/mL). (B) Homeostatic Model Assessment index. ap < 0.01 comparison with baseline (Wilcoxon test for paired samples), bp < 0.01 comparison with placebo (Mann-Whitney U test).
Clinical evaluation
The proportion of D-002-treated patients who reported clinical improvement of some symptoms (12/25, 48.0%) was significantly greater than in the placebo group (2/25, 8.0%; p < 0.001), more placebo patients (22/25, 88.0%) than D-002-treated patients (13/25, 52.0%) reported no change in their symptoms (p < 0.05), and only one placebo patient declared that the symptoms had worsened.
Safety and tolerability
D-002 (100 mg/day) given for 24 weeks was well tolerated by the patients. The treatment did not affect physical (bodyweight, pulse rate, arterial pressure) safety indicators, and did not modify significantly the remaining blood indicators (creatinine, bilirubin, prothrombin time; data not shown for simplicity). In addition, individual values remained within normal range. Only two placebo patients reported some degree of AE during the study (allergy and skin rash, respectively), which were causes of the two premature withdrawals.
DISCUSSION
Our data show that D-002 (100 mg/day) given for 24 weeks effectively reduced liver fat accumulation and IR in a group of patients with NAFLD. We observed a significant reduction in liver steatosis, HOMA index and insulin levels after 24 weeks of treatment, as well as a significant increase in plasma TAS, and symptom relief as compared to placebo patients.
Certain characteristics were overrepresented in the study subjects. Many were overweight (64.0%), had elevated serum TC (78.0%), hypertension (50.0%), elevated serum TG (40.0%), were smokers (28.0%) and were obese (24.0%) and the proportion of females surpassed that of males, consistent with the epidemiological characteristics reported for NAFLD [1,30-32]. Moreover, 24 (48.0%) patients had concomitant hypertension, serum TG ≥ 1.7 mmol/L; low HDL-C levels and fasting glucose ≥ 5.6 mmol/L, qualifying metabolic syndrome, a cluster of components including abdominal obesity, impaired glucose metabolism, dyslipidemia and arterial hypertension [33]. Nevertheless, since we did not measure waist circumference, a limitation of this study, we cannot confirm whether our patients were affected by this condition.
We did not use liver biopsy, the gold standard for the diagnosis and the only method able to differentiate simple NAFLD from NASH, and therefore we cannot rule out the possibility that this entity was present in some patients. Nevertheless, to avoid liver biopsy, one study proposed a NASH score based on cutoff values of noninvasive markers (BMI ≥ 47, ALT ≥ 32 IU/mL, AST ≥ 25 IU/mL, ALP ≥ 85 IU/mL, HOMA-IR ≥ 4, among others) in obese patients with liver steatosis [34,35]. In our study, only two patients with BMI ≥ 47 (1 placebo, 1 D-002-treated) had concomitant ALT, AST, ALP, and HOMA values greater than the proposed cutoff; these could indicate NASH.
The proportion of D-002-treated patients that achieved complete fatty liver regression assessed by ultrasonography (28%) in this study is promising, being slightly greater than that reported for 24 weeks of treatment with PUFA from seal oils (19.7%) [12]. The lack of a liver biopsy could hamper the relevance of our findings due to the subjective nature of the imaging methods used to diagnose NAFLD; i.e., ultrasound, computed tomography, and magnetic resonance imaging, all of which have high sensitivity and specificity for detecting steatosis, but are unable to differentiate NAFLD from NASH [36].
Nevertheless, despite its operator-dependence, abdominal ultrasound has been validated against histopathological specimens and other imaging methods in the diagnosis of liver steatosis [37]. It is thus suitable for the diagnosis and follow-up of patients with NAFLD because it is noninvasive, low-cost and widely available. In light of these facts and since this study was a pilot trial to assess the putative effects of D-002 on NAFLD, we selected ultrasound changes as a primary outcome because it is noninvasive, relatively simple and commonly used as the first approach to NAFLD diagnosis, meaning that the conditions of our study approximated those of routine clinical practice.
In addition, the randomized, double-blind, and placebo-controlled design of the study may reduce the impact of this subjective bias, since subjective mistakes should be equal between the two groups. Also, we included objective biochemical variables (IR markers, liver enzymes, TAS) as secondary efficacy outcomes, which contribute to the evaluation of the effects of D-002 on NAFLD patients.
Our data suggest that that D-002 (100 mg/day) effectively lowered IR in accordance with the significant decrease of the HOMA index (39.9%), an evidence-based surrogate measure of IR [38], and of the insulin (37%) levels after 24 weeks of treatment. In light of these facts, our results suggest the potential clinical relevance of the use of D-002 to improve IR in patients with NAFLD, an effect that could be explained by the action of triacontanol, the main component of D-002, which activates adenosine monophosphate (AMP)-kinase, likely leading to metabolic gene regulation and to AMP-mediated reduction of insulin secretion by pancreatic β-cells. This is similar to the effect of metformin, a well-known insulin-sensitizer [39].
Overall, the effect of D-002 on IR indicators (HOMA index, insulin levels) and TAS (an indicator of the antioxidant response) suggests its efficacy on the ultrasonographic improvement of liver fat accumulation [40]. In this study we evaluated the antioxidant effect of D-002 by assessing its effect on plasma TAS, a variable that allows estimation, as a whole, of the ability of the body's antioxidant systems to remove free radicals and ameliorate the damage they may cause [40]. This has been shown to be particularly reduced in obese children with NAFLD [41]. Therefore, the fact that D-002 increased TAS values indicates that it increased the antioxidant response in patients with NAFLD.
Currently, the cornerstone for NAFLD management is weight reduction, mainly a decrease in central obesity, and reversal of IR [8,9]. In our study, however, despite 23/24 placebo and 21/24 D-002-treated patients being advised to follow a specific diet, no body weight reduction was observed during the trial [8,9]. Thus, the improvement in liver fat accumulation, HOMA index and insulin levels observed in the D-002-treated group seems not to be strongly influenced by dietary intervention, but rather is attributable to D-002 treatment.
Transaminase levels did not change significantly during the treatment. Although this seems to disagree with our efficacy hypothesis, it could be related to the fact that most patients had normal or relatively low baseline transaminase levels, the most frequent being high GGT values (≤ 55 UI/L) (GGT, 41/50, 82%; ALT, 47/50, 94.0%; AST, 50/50, 100%).
In conclusion, treatment of NAFLD with D-002 seems to be effective and safe, and may ameliorate liver fat accumulation and clinical evolution of subjects, may reduce the HOMA index and insulin levels, and may increase the antioxidant response of the body, as assessed by plasma TAS. Nevertheless, further studies are needed to confirm the efficacy, safety, and tolerability of D-002 in NAFLD patients.
KEY MESSAGE
1. Oral administration of D-002 (100 mg/day) for 6 months may ameliorate liver fat accumulation and insulin resistance, meanwhile improve clinical evolution and antioxidant response in subjects with nonalcoholic fatty liver disease (NAFLD).
2. D-002 seems to be safe and well tolerated in subjects with NAFLD.
Notes
No potential conflict of interest relevant to this article is reported.