Renal adverse effects of sunitinib and its clinical significance: a single-center experience in Korea
Article information
Abstract
Background/Aims
Sunitinib is an oral multitargeted tyrosine kinase inhibitor used mainly for the treatment of metastatic renal cell carcinoma. The renal adverse effects (RAEs) of sunitinib have not been investigated. The aim of this study was to determine the incidence and risk factors of RAEs (proteinuria [PU] and renal insufficiency [RI]) and to investigate the relationship between PU and antitumor efficacy.
Methods
We performed a retrospective review of medical records of patients who had received sunitinib for more than 3 months.
Results
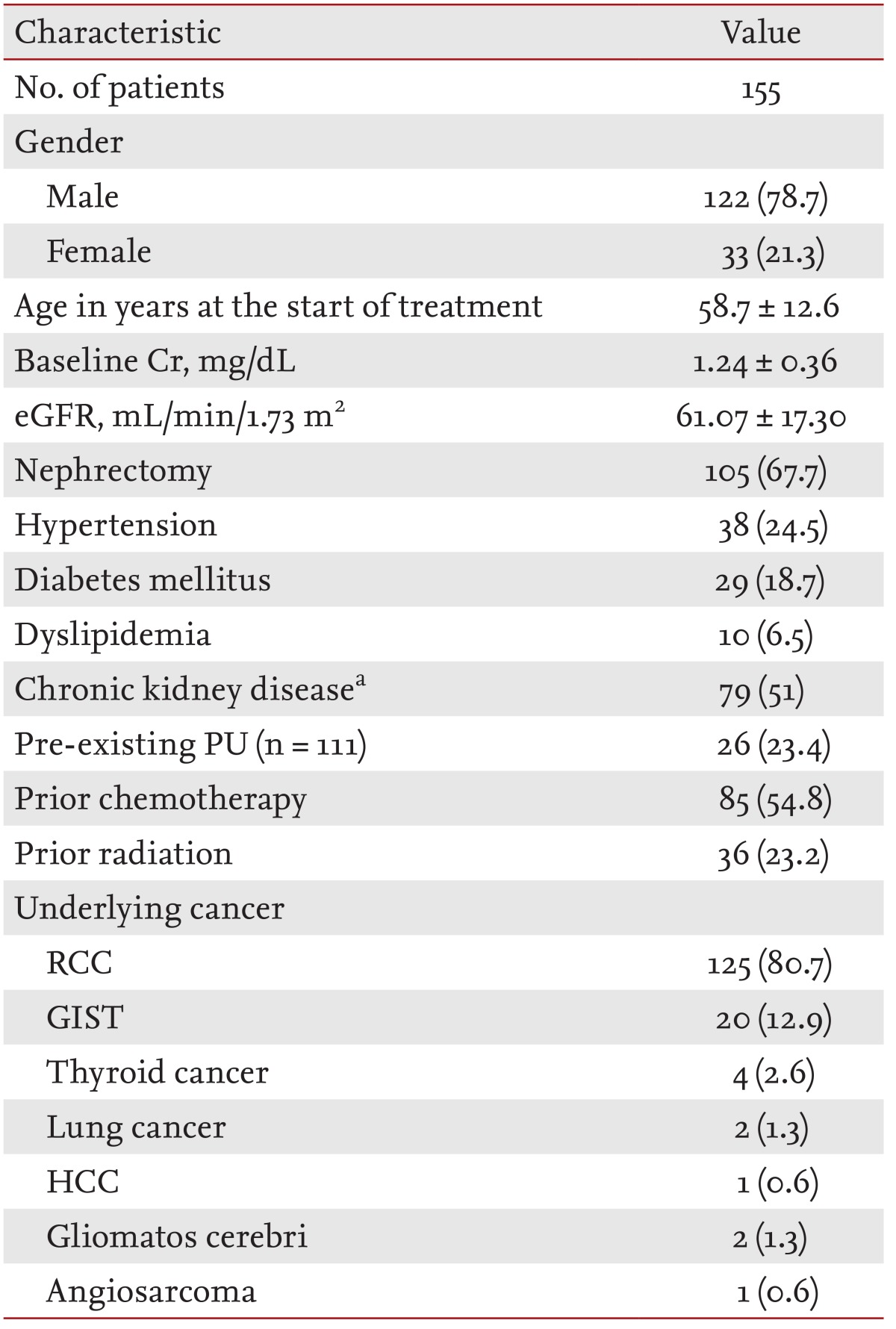
One hundred and fifty-five patients (mean age, 58.7 ± 12.6 years) were enrolled, and the mean baseline creatinine level was 1.24 mg/dL. PU developed in 15 of 111 patients, and preexisting PU was aggravated in six of 111 patients. Only one patient developed typical nephrotic syndrome. Following discontinuation of sunitinib, PU was improved in 12 of 17 patients but persisted in five of 17 patients. RI occurred in 12 of 155 patients, and the maximum creatinine level was 3.31 mg/dL. RI improved in two of 12 patients but persisted in 10 of 12 patients. Risk factors for PU were hypertension, dyslipidemia, and chronic kidney disease. Older age was a risk factor for RI. The median progression-free survival was significantly better for patients who showed PU.
Conclusions
The incidence of RAEs associated with sunitinib was lower than those of previous reports. The severity of RAEs was mild to moderate, and partially reversible after cessation of sunitinib. We suggest that blood pressure, urinalysis, and renal function in patients receiving sunitinib should be monitored closely.
INTRODUCTION
Recently, inhibition of tumor angiogenesis has been the focus of anticancer treatments [1]. Members of the vascular endothelial growth factor (VEGF) family and their receptors, tyrosine kinase receptors, were identified as regulators of physiological or pathological angiogenesis [1-3]. From a pathological point of view, overexpression of VEGF family members was found in hypervascular solid tumors and might be associated with tumor angiogenesis, growth, and metastasis [4]. These findings have led to the development of VEGF-targeted agents, either as monoclonal antibodies against VEGF (e.g., bevacizumab) or small tyrosine kinase inhibitors (TKIs) [5], and these agents are now widely used for the treatment of various tumors. Sunitinib is approved for the first-line treatment of advanced and/or metastatic renal cell carcinoma (mRCC) and is effective in the treatment of advanced gastrointestinal stromal tumor (GIST) after failure of imatinib treatment [6-8]. Common toxicities associated with sunitinib are diarrhea, fatigue, anorexia, nausea, mucosal inf lammation, hand-foot syndrome, and laboratory abnormalities, including thrombocytopenia, neutropenia, and anemia. Physicians prescribing sunitinib are accustomed to monitoring those common toxicities [1,6]. The reported renal adverse effects (RAEs) of sunitinib were hypertension (HTN), proteinuria (PU), renal insufficiency (RI), and thrombotic microangiopathy [2,6,7,9-13]. Among patients receiving sunitinib, the incidence of all grade increased creatinine levels is 12.4% to 65.6%, with the highest incidence in patients with mRCC in the phase III study by Motzer et al. [14], and the lowest incidence in GIST patients in the phase III trial by Demetri et al. [7]. Renal function was deteriorated in 57% of the patients with preexisting RI at the start of TKI therapy [9]. Although PU, which is sometimes in the nephrotic range, has also been reported [12,13], its incidence and clinical course have not been fully described. Because sunitinib is administered orally, and initial dose adjustment according to renal function is not recommended, some physicians might not be concerned over the RAEs of these agents. Moreover, sunitinib-associated RAEs are not readily identifiable upon physical examination if the physician is not concerned about those complications. Zhu et al. [6] recently reported the incidence and relative risk of HTN and renal dysfunction in a meta-analysis. However, most of the data concerning the incidence of RAEs originate from well-controlled clinical trials, and so their incidence might be different in routine clinical practice. Moreover, little is known regarding the risk factors for development of RAEs in patients prescribed sunitinib.
Development of HTN might be a biomarker of antitumor efficacy and effective VEGF signaling inhibition [10,15,16]. Therefore, the development of PU resulting from inhibition of the VEGF signaling pathway may also be a surrogate marker of antitumor activity.
The aim of this retrospective study was to determine the incidence and risk factors of RAEs in patients who were administered sunitinib in routine clinical practice and to investigate the relationship between PU and antitumor efficacy in patients treated with sunitinib.
METHODS
Subjects and study design
Patients, aged 18 years or older, who had received sunitinib for at least 3 months, were selected from the Seoul National University Hospital electronic records from November 2005 to May 2011. Patients with obstructive uropathy and unavailable urinalysis and serum creatinine (sCr) follow-up data were excluded from the study. Sunitinib was administered daily on a 4-week-on and 2-week-off schedule during the 6-week period following initiation of therapy. The study was approved by the Institutional Review Board of Seoul National University Hospital.
Measurements and definitions
Patients' data were collected retrospectively from a review of the electronic medical records. All recorded urinalysis, sCr measurements, comorbid conditions such as HTN, diabetes mellitus, dyslipidemia, chronic kidney disease (CKD), and underlying cancers and medication profiles were reviewed. The presence of HTN, diabetes mellitus, and dyslipidemia at baseline was determined by a self-reported history or use of antihypertensive medications, hypoglycemic agents, and lipid-lowering agents, respectively. HTN was also defined as a systolic blood pressure level > 140 mmHg or a diastolic blood pressure level > 90 mmHg at the physical examination. Underlying CKD was defined as an estimated glomerular filtration rate (eGFR) < 60 mL/min/1.73 m2 for more than 3 months before the start of sunitinib. PU was defined as ≥ 1+ albuminuria on a dipstick test or a urine protein creatinine ratio (PCR) > 0.3. Aggravation of preexisting PU was defined as an increase in albuminuria by more than two grades on the dipstick test or a more than 2-fold increase in the urine PCR. Both newly developed and aggravated PU were regarded as PU for the purposes of analysis. To determine the severity of PU, we used the National Cancer Institute PU grading system (grade 1, 1+ PU, urinary protein < 1.0 g/24 hr; grade 2, 2+ PU, 1.0 to 3.4 g/24 hr; grade 3, > 3.5 g/24 hr) [17]. RI was defined as an increase ≥ 50% or ≥ 0.5 mg/dL in sCr levels; RI with identifiable causes was not included. Improvement of PU and RI was defined as normalization or decrease to the baseline PU and eGFR levels. We excluded transient PU and RI that developed due to other identified specific causes such as infection, drugs other than sunitinib, and gastrointestinal bleeding. Progression-free survival (PFS) was defined as the time from initiation of sunitinib therapy until disease progression or death from any cause. Disease progression was assessed using the Response Evaluation Criteria in Solid Tumors [18].
Statistical analysis
All analyses were performed using SPSS version 17.0 (SPSS Inc., Chicago, IL, USA). Data are presented as means ± SD for continuous variables and as proportions for categorical variables. Differences in continuous variables were analyzed by t tests, and by chisquare tests for categorical variables. The effects of sunitinib on the risks of RI and PU were evaluated by binary logistic regression. PFS was estimated using the Kaplan-Meier survival analysis method and compared using the Breslow-Day test. A p value less than 0.05 was deemed to indicate statistical significance.
RESULTS
Characteristics of the subjects
One hundred and fifty-five patients were enrolled in the present study. Table 1 summarizes the baseline patient characteristics. The mean age of the patients was 58.7 ± 12.6 years, and male patients were predominant (male, 78.7%). The baseline eGFR and mean baseline sCr level of the patients were 61.07 ± 17.30 mL/min/1.73 m2 and 1.24 ± 0.36 mg/dL, respectively. At the start of sunitinib therapy, 24.5% of the patients had HTN, 18.7% had diabetes mellitus, and 51% had CKD. Underlying diseases of the patients were renal cell carcinoma (80.7%), GIST (12.9%), thyroid cancer (2.6%), lung cancer (1.3%), gliomatosis cerebri (1.3%), hepatocellular carcinoma (0.6%), and angiosarcoma (0.6%).
RAEs (PU and RI)
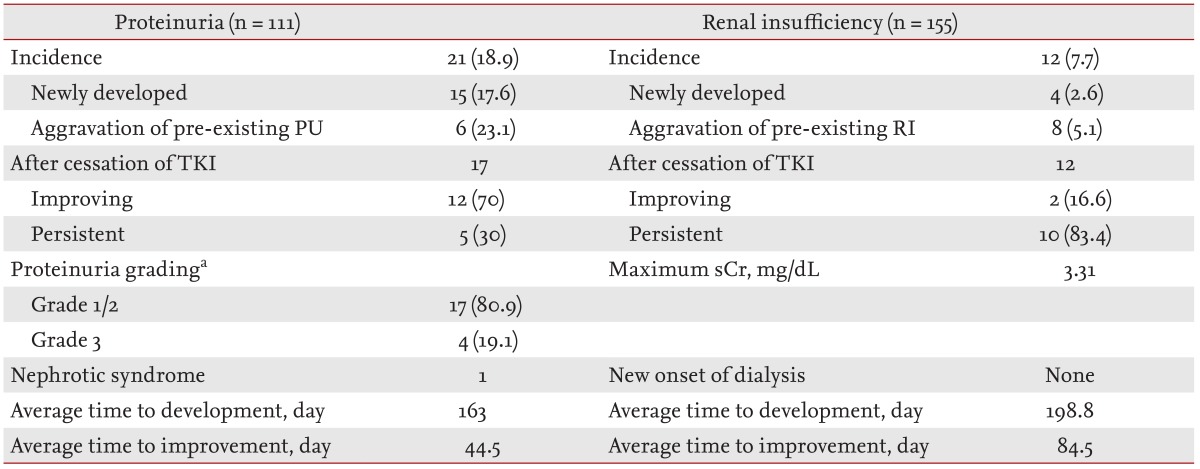
The incidences and clinical courses of PU and RI after initiation of sunitinib are shown in Table 2. Of the 155 subjects, 111 had baseline urinalysis data available, and the incidence and risk factors of PU were analyzed in those patients. At initiation of sunitinib therapy, 23.4% of the patients had pre-existing PU (Table 1), and newly developed or aggravated PU was observed in 21 of 111 patients (18.9%). PU developed in 15 of 85 patients (17.6%), and preexisting PU was aggravated in six of 26 patients (23.1%) after initiation of sunitinib therapy (Table 2). When patients with PU were grouped into grades 1 to 3 according to the National Cancer Institute's PU grading system [17], four patients showed grade 3 PU, but symptomatic nephrotic syndrome developed in only one patient. Among the patients with PU, 17 patients (80.9%) discontinued the medication; PU improved in 12 of these 17 patients (70%) but persisted in five (30%). The dosage of sunitinib was reduced in the remaining four patients, among whom PU persisted in three and improved in one. PU developed at a mean of 163 days after initiation of treatment.
RI occurred in 12 of the 155 patients (7.7%), and the maximum sCr level during treatment was 3.31 mg/dL. The average time to the development of RI from the start of the medication was 199 days. All patients in whom renal function deteriorated stopped taking the medication. RI improved in two of the 12 patients (16.6%) but persisted in 10 patients (83.4%). However, no patient had RI sufficiently severe to require hemodialysis (Table 2).
Baseline characteristics of patients with RAEs
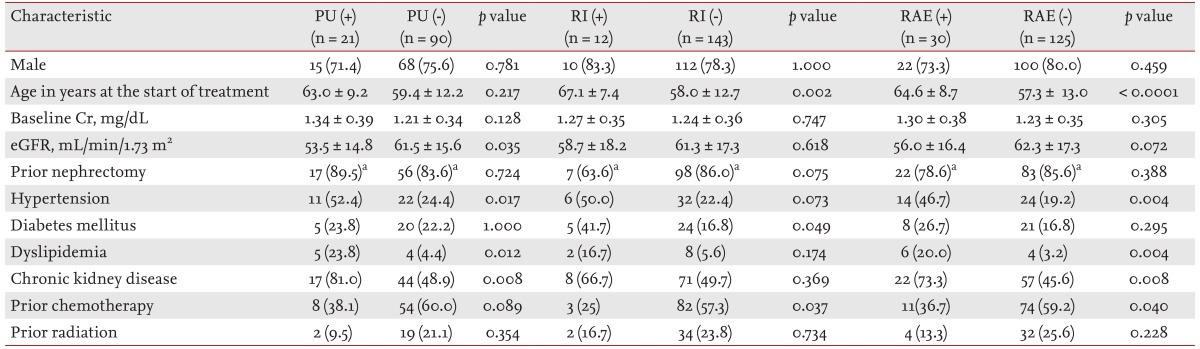
The patients with PU, compared with those without PU, showed a lower eGFR (53.5 ± 14.8 mL/min/1.73 m2 vs. 61.5 ± 15.6 mL/min/1.73 m2, respectively; p = 0.035) and higher incidences of HTN, dyslipidemia, and CKD (52.4% vs. 24.4%, p = 0.017; 23.8% vs. 4.4%, p = 0.012; 81.0% vs. 48.9%, p = 0.008, respectively). Older age (67.1 ± 7.4 years vs. 58.0 ± 12.7 years; p = 0.002), a higher incidence of diabetes mellitus (41.7% vs. 16.8%; p = 0.049), and a lower proportion of prior adjuvant chemotherapy (25% vs. 57.3%; p = 0.037) were observed in patients who developed RI during treatment. Among the patients with RAEs (PU or RI), older age (64.6 ± 8.7 years vs. 57.3 ± 13.0 years; p < 0.0001), higher incidences of HTN, dyslipidemia, and CKD (46.7% vs. 19.2%, p = 0.004; 20.0% vs. 3.2%, p = 0.004; and 73.3% vs. 45.6%, p = 0.008, respectively), and a lower proportion of prior adjuvant chemotherapy (36.7% vs. 59.2%; p = 0.040) were identified (Table 3).
Risk factors for PU and RI with sunitinib therapy
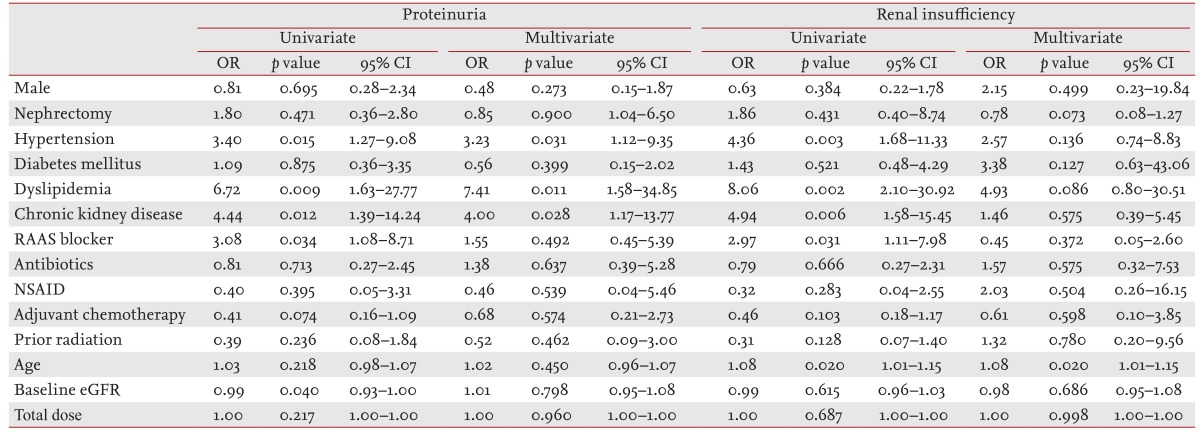
We analyzed independent risk factors by binary logistic regression adjusted for age, gender, baseline eGFR, total sunitinib dose, HTN, diabetes mellitus, dyslipidemia, CKD, renin-angiotensin-aldosterone blockers, antibiotics, nonsteroidal anti-inflammatory drugs, adjuvant chemotherapy, and prior radiation. Risk factors for PU were HTN, dyslipidemia, and CKD at initiation of sunitinib therapy (odds ratio [OR], 3.23, 95% confidence interval [CI], 1.12 to 9.35, p = 0.031; OR, 7.41, 95% CI, 1.58 to 34.85, p = 0.011; OR, 4.00, 95% CI, 1.17 to 13.77, p = 0.028, respectively). Older age was significantly associated with development of RI (OR, 1.08; 95% CI, 1.01 to 1.15; p = 0.020) (Table 4).
Relationship between PU and PFS
Because few patients suffered from non-mRCC, including GIST and thyroid cancer, and those with mRCC were more homogeneous, the relationship between PFS and PU was analyzed only in patients with mRCC. The median PFS was significantly longer for patients in whom PU developed or was aggravated (median PFS, 245 days, 95% CI, 150 to 340 vs. median PFS, 469 days, 95% CI, 198 to 740, p = 0.020) (Fig. 1).

Progression-free survival (PFS) according to proteinuria status. Kaplan-Meier estimates of PFS according to proteinuria status. The median PFS was significantly longer for patients who had development or aggravation of proteinuria (PU) (median PFS, 245 days, 95% confidence interval [CI], 150 to 340 vs. median PFS, 469 days, 95% CI, 198 to 740; p = 0.020).
DISCUSSION
Sunitinib is an oral multitargeted small TKI used mainly in the treatment of mRCC, and its indication is being extended. Sunitinib treatment-related AEs, such as diarrhea, fatigue, anorexia, nausea, mucosal inflammation, hand-foot syndrome, HTN, thrombocytopenia, neutropenia, and anemia were described in early studies, and most physicians are aware of those adverse effects. Recently, RAEs associated with sunitinib, including severe cases of nephrotic syndrome, thrombotic microangiopathy, and acute kidney injury requiring hemodialysis, have been reported [12,13,19]; however, their incidence and clinical significance in routine clinical practice are uncertain.
The present retrospective analysis evaluated the incidence and risk factors of PU and RI associated with sunitinib treatment. We also analyzed the relationship between PU and PFS during sunitinib treatment.
Although sunitinib-associated nephrotoxicity was not reported in the initial clinical trials, recent meta-analyses have revealed that an increased incidence of RI in patients receiving TKI. In patients with mRCC treated with sunitinib, the incidence of all grades of creatinine increase was 65.6% (95% CI, 60.6 to 70.2); in patients with GIST, the incidence was 12.4% (95% CI, 8.5 to 17.7) [6]. Thus, treatment with TKI is likely associated with significant renal morbidity. However, the causes of RI were not provided in this meta-analysis, and so renal dysfunction resulting from causes other than sunitinib, such as infection and drugs, could have been included. The etiology of sunitinib-related renal dysfunction is poorly understood. One possible explanation is that uncontrolled HTN causes renal injury. In the present study, eight of 12 patients with RI had new-onset or aggravated HTN, which lends support to this hypothesis. Conversely, Zhu et al. [6] proposed that kidney injury might be caused by direct toxicity of sunitinib to glomeruli and renal tubules due to its anti-VEGF effects. Additionally, Kim et al. [20] reported that anti-VEGF therapy induced renal injuries, such as inflammation, glomerulosclerosis, and tubulointerstitial fibrosis, in a diabetic mouse model. The therapy was associated with down-regulation of nitric oxide production and up-regulation of hypoxia-induced oxidative stress, but had no effect on blood pressure [20]. The incidence of RI in the present study was 7.7%, which is lower than that of previous reports, including Korean studies [6,9,13,21]. Possible explanations are as follows. First, the current study included patients with underlying diseases other than mRCC. Second, we included only patients with acute kidney injury, the causes of which were known to be associated with sunitinib. Third, the definition of RI differed from those in previous studies [9,21]. We found volume depletion and use of nephrotoxic drugs to be causes of renal dysfunction, which were reversible, and so not categorized as RI. Although 10 of 12 patients with RI had persistent renal dysfunction after cessation of medication, their renal function did not deteriorate progressively, and none required renal replacement therapy. RI in the present study was mild to moderate, and its incidence was not high. However, because the primary recipients of sunitinib treatment are elderly patients with a small nephron mass due to prior nephrectomy [6], renal function should be monitored carefully [6,13,22]. Although sunitinib does not commonly lead to severe renal dysfunction, clinicians should be aware of the potential RAEs when managing cancer patients treated with sunitinib.
The incidence of sunitinib-associated PU was 18.9%, and sunitinib led to all-grade PU with a low frequency of high-grade PU (grade 1/2, 80.9%; grade 3, 19.1%). This finding is comparable to that of a recent study that included Korean patients [21]. Most patients with PU, except one, were asymptomatic, and PU was resolved by drug discontinuation or dose reduction in 62% of the patients. PU was reversed, at least partially, after drug discontinuation in most cases, and treatment was resumed without further worsening of PU in some patients. Sunitinib-induced heavy PU may require underlying renal lesion [2]. A small number of patients showed higher levels of PU, edema, HTN, and hyponatremia, and Patel et al. [23] reported those cases as having preeclampsia-like syndrome. Jhaveri et al. [13] also reported four patients with various degrees of HTN, PU, thrombotic microangiopathy, and acute and chronic kidney injury. Thrombocytopenia and/or a history of a solitary kidney prohibited a kidney biopsy in most cases, but the underlying pathology in a few cases was biopsy-proven to be thrombotic microangiopathy and acute interstitial nephritis [24-26]. The mechanism of renal dysfunction and PU in patients receiving antiangiogenesis agents is yet to be elucidated, but it might be associated with the functions and localization of VEGF and VEGF receptor [2]. One possible mechanism of anti-VEGF-associated PU is elevated intraglomerular pressure, similar to that in systemic HTN [2,27]. Izzedine et al. [27] reported that PU is partially correlated with HTN: 54% of patients with grade 2/3 HTN developed PU grade 2/3, and 16% of patients with grade 0/1 HTN developed PU during bevacizumab treatment. Another possible mechanism is renal changes, including reduction of endothelial fenestration in glomerular capillaries, endotheliosis, and foot process effacement resulting from loss of nephrin expression, while VEGF expression is reduced by anti-VEGF therapies [2,27].
Four patients had both PU and RI in the present study. Although biopsy could not be performed due to a solitary kidney, none of them showed features of thrombotic microangiopathy. Moreover, PU improved in two of the four patients, and RI persisted; however, the renal function of all four patients did not show progressive deterioration after cessation of sunitinib treatment.
The development of HTN in patients using bevacizumab and sunitinib may be a surrogate marker of antitumor efficacy and effective VEGF signaling inhibition [10,15,16,28-31]. We examined the association between PU and median PFS by Kaplan-Meier analysis. PFS was significantly better in patients with new-onset or aggravated PU (median PFS, 469 days, 95% CI, 198 to 740 vs. median PFS, 245 days, 95% CI, 150 to 340; p = 0.020). This is to our knowledge the first study to evaluate the relationship between development of PU and PFS with VEGF receptor inhibitor therapy. Our data suggest that PU may be a surrogate marker of antitumor activity. Therefore, development or aggravation of PU may not require discontinuation of the drug if not in the nephrotic range. However, there is still no consensus regarding the acceptable PU level in patients with anti-VEGF-associated PU. In the case of heavy PU, blockade of the renin-angiotensin-aldosterone system is first considered, and sunitinib may be continued with close monitoring. PU and HTN could originate from the class effects of sunitinib, which might explain our finding [11]. Additional, larger prospective studies are warranted to elucidate the relationship between PU and antitumor activity because of the small number of patients and retrospective nature of the present study.
The current study has several limitations. First, missing data were considerable. Twenty-two percent of the subjects had no baseline urinalysis data. However, unlike previous clinical trials, we thoroughly reviewed the medical records to minimize errors. Second, we were unable to obtain complete blood pressure data, which might affect PU and/or RI. Because blood pressure was recorded in only 58% of the initially normotensive patients in the present study, whether blood pressure is a confounding factor for the development and/or aggravation of PU remains unknown. PU occurred in 14 of 21 patients without development and/or aggravation of HTN, thus, PU might occur regardless of HTN in a considerable number of patients. However, Machado et al. [32] reported that sunitinib-induced HTN, PU, interstitial expansion, and glomerulosclerosis were not observed in normal rats but were found in a rat model with kidney damage. It is possible that patients with HTN have a higher probability of having renal damage prior to therapy and could be at greater risk of the development of PU and RI. The effect of blood pressure on PU and RI with anti-VEGF agents requires validation in well-designed prospective studies. More importantly, we found that many physicians did not pay attention to blood pressure monitoring despite the recommendations [13]. Third, this is a singlecenter study of a Korean population, and differences in clinical practice patterns might influence the results [21]. Therefore, our findings cannot be generalized.
In conclusion, the incidence of RI associated with sunitinib was lower (7.7%) than that of previous reports, while the incidence of PU was comparable (18.9%) to those of other studies. The independent risk factors for PU were HTN, dyslipidemia, and CKD; only older age was associated with development of RI. PU was associated with a longer PFS. The severity of most RAEs was mild to moderate, and partially reversible after discontinuation of the therapy in most cases. However, due to the potential for RAEs, urinalysis, kidney function, and measurement of blood pressure should be conducted at initiation of sunitinib therapy and monitored regularly. Patients who had significant risk factors, including underlying HTN, dyslipidemia, CKD, and older age, require more careful monitoring for the occurrence of RAEs. Sunitinib may be continued for mild to moderate PU and RI with close monitoring because of its association with better oncological outcomes.
KEY MESSAGE
1. The incidences of renal insufficiency and proteinuria associated with sunitinib were 7.7% and 18.9%, respectively.
2. Patients with hypertension, dyslipidemia, chronic kidney disease, and older age were more vulnerable to sunitinib-induced renal adverse effects.
3. Baseline urinalysis, kidney function tests, and blood pressure measurement should be performed at initiation of sunitinib therapy and monitored regularly.
Notes
No potential conflict of interest relevant to this article is reported.