Relationship between duration of hospital-acquired acute kidney injury and mortality: a prospective observational study
Article information
Abstract
Background/Aims
New definitions of acute kidney injury (AKI) have recently emerged. Some studies have suggested that duration of AKI is an additional predictive parameter for mortality. Here, we evaluated whether AKI duration was predictive of long-term mortality in patients with hospital-acquired acute kidney injury (HAAKI).
Methods
We prospectively enrolled patients who developed HAAKI at an urban university hospital, from September 2007 to August 2008 and followed them until December 2011. Patients were divided into two groups by duration of the AKI (1 to 5 days vs. ≥ 6 days), and long-term mortality was compared.
Results
HAAKI developed in 1.2% of patients during the enrollment period. The median follow-up period was 240 days (interquartile range, 53 to 1,428). In 42.3% of patients (n = 52), the AKI lasted 1 to 5 days, while it lasted ≥ 6 days in 57.7% (n = 71). Survival analysis showed that a longer duration of AKI increased the risk of death. Long-term survival was significantly different in the two groups.
Conclusions
The duration of AKI influenced mortality rates in hospitalized patients. Thus, AKI duration is a parameter affecting mortality in HAAKI.
INTRODUCTION
Acute kidney injury (AKI) occurs commonly in hospitalized patients. It is associated with an increased risk of mortality and length of hospital stay (LOS) [1,2,3,4,5,6]. The spectrum of AKI ranges from non-symptomatic, with minimal elevations of serum creatinine, to a completely anuric state. Knowledge and understanding of AKI epidemiology is important in dealing with this syndrome. There have been several consensus statements proposed in attempts to define and stage AKI for use in practice and research. In 2004, the Acute Dialysis Quality Initiative group proposed a consensus with definition and staging of acute renal failure known as the 'RIFLE' criteria (risk, injury, failure, loss, and end-stage kidney disease) [7]. The RIFLE criteria were refined further by taking into account a smaller change in serum creatinine according to the Acute Kidney Injury Network (AKIN) classification of 2007 [8]. More recently, the Kidney Disease/Improving Global Outcomes (KDIGO) AKI workgroup proposed a modified definition that harmonized differences between the RIFLE and AKIN definitions [9]. According to these criteria, the severity of AKI is based on degree of creatinine elevation and decrease in urine output [7,8,9].
Numerous epidemiologic studies have supported the notion that AKI severity influences prognosis [10,11,12,13,14]. Additionally, in a few recent studies, duration of AKI has been suggested as an important factor affecting the prognosis. [15,16,17,18]. These studies have argued that AKI duration is an additional clinical parameter that should be included in the criteria for determining the severity of AKI. One study observed critically ill patients admitted to the intensive care unit (ICU), and other studies observed all hospitalized patients with conditions including community-acquired acute kidney injury (CAAKI) and some specific surgical patients. However, the idea that AKI duration affects prognosis needs to be validated in a variety of cohorts. Although AKI is commonly found in hospitalized patients, almost 60% of AKI occurs in patients who have not received care in an ICU [19]. Hospital-acquired acute kidney injury (HAAKI) developed during hospitalization can significantly affect patient outcomes and can even lead to death [18,19,20,21]. Also, the characteristics of HAAKI can differ, according to various hospital settings [22]. Thus, we conducted this study to evaluate whether the duration of AKI can predict long-term mortality in HAAKI.
METHODS
Study design
We enrolled HAAKI patients prospectively and collected clinical data on them. All patients were admitted to a tertiary 700-bed academic medical center in Seoul, Korea. Patients were enrolled from September 2007 to August 2008 and data on their progress, renal complications, functional impairment, and recovery were collected. We published the hospital mortality according to the AKIN criteria [13]. The patients were followed until December 2011 to evaluate long-term mortality. Some patients were admitted on more than one occasion during the study period and each admission was considered a separate case.
Ethics approval was obtained from the Soonchunhyang University Hospital Institutional Review Board. Patient consent was not obtained for this prospective observational study because no intervention by the study investigators could have affected patient care.
We excluded patients younger than 18 years, patients with previously diagnosed end-stage renal disease or renal transplantations, and patients with a LOS less than 48 hours. Because late-stage patients' kidney function aggravation, such as in terminal cancer patients, was considered to lead to death, it was not categorized as a treatment-related complication. Serum creatinine levels were reviewed daily for each patient. When serum creatinine was elevated, we reviewed the patient's medical records and gathered laboratory blood data.
Definitions
Serum creatinine was used to diagnose AKI and to determine the AKIN stage. Patients were classified according to the worst stage of injury after admission. HAAKI was defined as an abrupt reduction in kidney function, signified by an absolute increase in serum creatinine of ≥ 0.3 mg/dL from baseline or an increase in the serum creatinine ≥ 50% within 48 hours (AKIN criteria). Patients whose serum creatinine was elevated within 48 hours after admission were excluded, to exclude CAAKI. Baseline creatinine was determined in two ways. For patients who had a prior serum creatinine level in their records, baseline creatinine was defined as the level measured upon discharge from the most recent hospitalization or from the most recent clinic visit. For patients with no available record, the baseline level was defined as the lowest serum creatinine level during hospitalization. The duration of AKI was defined as the number of days that AKI was present from the first day the patient met the AKI criteria until they no longer did. There is currently no consensus on the categorization of AKI duration. In our study, we split patients into two groups based on AKI duration. We chose the cutoff duration such that the two groups were evenly sized. For our patient population, this resulted in one group for those with AKI for 1 to 5 days and another for patients with AKI lasting at least 6 days. The severity of the AKI was classified using the AKIN staging criteria [8]. We did not consider urine output in our data analysis. Recovery from AKI was defined as a reduction of serum creatinine to baseline levels or within 0.3 mg/dL above baseline level within 3 months after AKI.
Demographic data and clinical and biochemical profiles
We obtained information on patient demographics and initial laboratory values, including hematocrit, albumin, serum creatinine, total cholesterol, C-reactive protein (CRP), LOS, and follow-up duration. Creatinine concentrations were determined by the Jaffe reaction with a Hitachi 7600-110 autoanalyzer (Tokyo, Japan). Also, the Charlson comorbidity index (CCI) score was used to compare comorbidity status at the time of admission [23].
Patient mortality: data source
The primary outcome was mortality. We referenced medical records for patients who could be followed, and for those where there was no direct follow-up, data from the National Health Insurance Corporation (NHIC) system was used.
Statistical analysis
Baseline characteristic comparisons across groups were performed using analysis of variance for continuous variables and chi-square tests for categorical variables. Data are presented as means ± standard deviation, while nominal data are shown as percentages. Variables with a non-normal distribution are expressed as medians (interquartile range [IQR]). CRP, LOS, and follow-up days for the two AKI duration groups were compared using the Kruskal-Wallis test. The survival rates were calculated using the Kaplan-Meier method. The log-rank test was used to compare survival among groups based on AKI duration or AKIN stage. The hazard ratio (HR) and 95% confidence intervals for mortality rates were calculated using the Cox proportional hazard analysis after adjustment for potential confounders. All statistical analyses were performed with the SPSS version 16.0 (SPSS Inc., Chicago, IL, USA). A two-sided p < 0.05 was considered to indicate statistical significance.
RESULTS
Patient characteristics
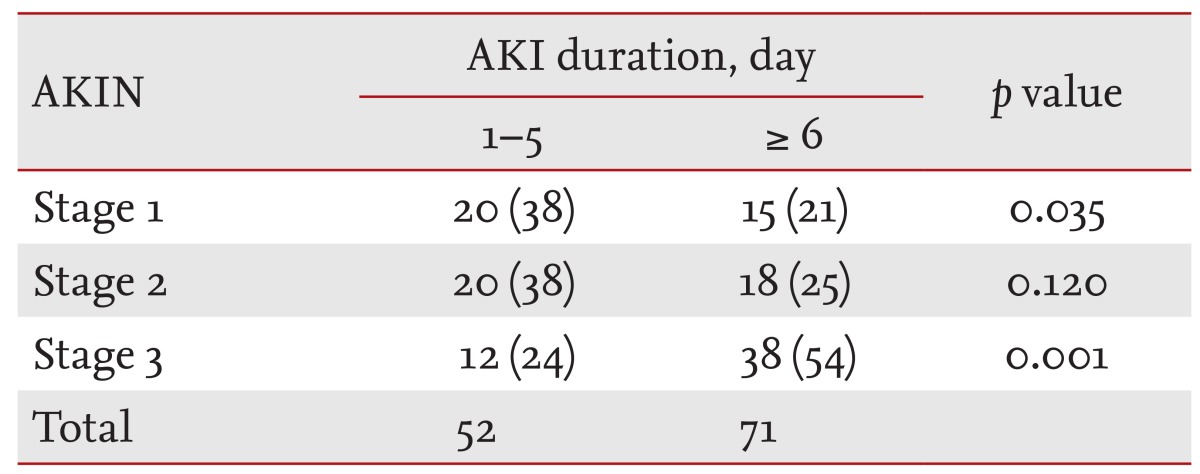
During the study period, 1.2% of patients (123/10,250) exhibited HAAKI. The mean age was 64.8 ± 13.5 years with a male predominance of 61%. Among the 123 HAAKI patients, 26.8% (n = 33) were admitted for surgical problems and 42.3% (n = 52) were admitted to the ICU. Prerenal causes of AKI were present in 26% and there was no postrenal AKI. Surgery-related AKI was present in 28.5% (n = 35), drug (angiotensin receptor blocker, angiotensin converting enzyme inhibitor, chemotherapy, diuretics, nonsteroidal anti-inflammatory drug)-induced AKI in 25.2% (n = 32), and contrast-induced AKI in 7.3% (n = 9). The median number of days after admission before a patient was diagnosed with AKI was 14 days (IQR, 7 to 27). HAAKI patients were followed until December 2011 and the median follow-up duration was 240 days (IQR, 53 to 1,428). Patients were categorized into two AKI duration groups and three AKIN stage groups. AKI lasted for 1 to 5 days in 42.3% of the patients, while 57.7% had AKI for 6 days or more. In terms of staging, 28.5% were categorized as AKIN stage 1, 20.9% as stage 2, and 40.7% as stage 3. There was no difference between the AKI duration or stage groups in terms of age, gender, body mass index, hematocrit, albumin, creatinine, total cholesterol, CRP, LOS, or underlying disease. The average CCI score upon admission was 2.5, with no differences between any of the groups. Baseline characteristics according to AKI duration are shown in Table 1, while a breakdown of AKI incidence by stage and duration is shown in Table 2.
Outcomes and long-term mortality
There was no difference in the LOS between the AKI duration and AKIN stage groups. Among the 123 patients with HAAKI, 16.2% (n = 20) were treated with renal replacement therapy. Three of them developed-end stage renal disease and required maintenance hemodialysis. In terms of death, 34.1% (n = 42) died during their hospital stays and 63.4% (n = 78) died during the follow-up period. In a multivariate survival analysis, stage 3 AKI was associated with an increased risk for death during long-term follow-up (p = 0.023).
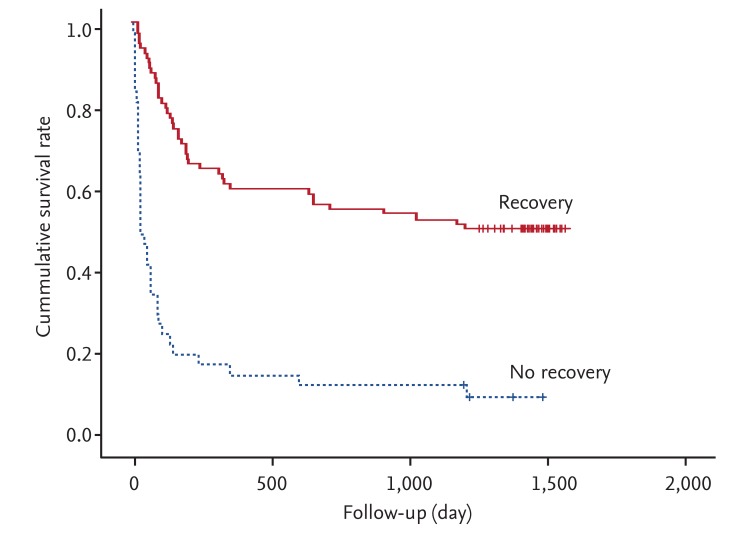
Fig. 1 shows the overall survival curves for the AKI duration groups. Patients with longer duration (≥ 6 days) of AKI showed a higher mortality than those with shorter duration (1 to 5 days; p = 0.014). Unadjusted HR for overall survival was 1.79 for longer duration AKI versus shorter duration. After adjusting for multiple confounding factors including age, gender, CCI, and recovery from AKI, the adjusted HR for overall survival was 1.74 for longer duration AKI versus shorter duration (Table 3). The proportion of patients who recovered from AKI was significantly different between the two duration groups: 98% recovered in the 1 to 5-day group, while only 44% recovered in the ≥ 6-day group (p < 0.0001). Also, long-term survival was significantly better for those who recovered than those who did not (p < 0.0001) (Fig. 2).
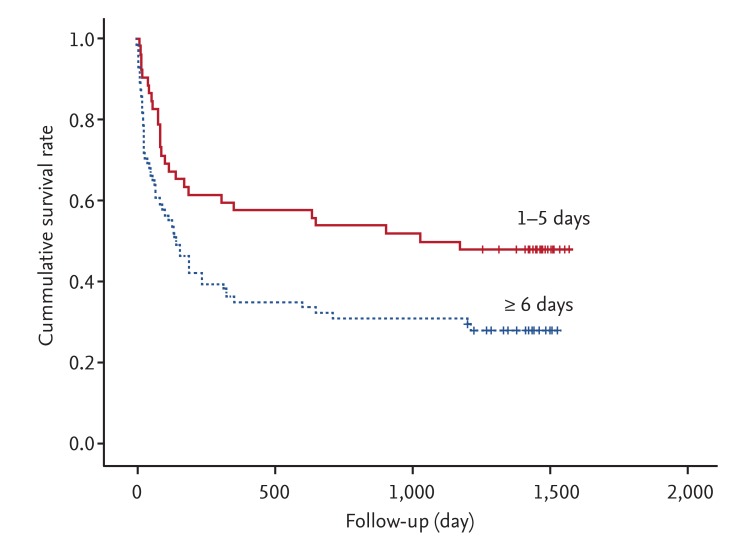
Kaplan-Meier survival plots by acute kidney injury duration. The overall survival rates of the two groups differed significantly (p = 0.014, log-rank test).
DISCUSSION
We found that the duration of AKI was associated with long-term mortality in AKI that developed during hospitalization. To our knowledge, this is the first reported study to validate the prognostic value of AKI duration in HAAKI patients. The longer duration group (≥ 6 days) was less likely to recover from HAAKI. Also, long-term mortality was significantly higher in those who did not recover from AKI than those who did. After adjusting for confounding factors including recovery from AKI, AKI duration was a significant factor for mortality.
The incidence of HAAKI has been increasing over the past several decades. The reasons for this increased incidence are not entirely clear. Comorbidities, decreased renal perfusion, medications, surgery, and the use of radiographic contrast media during treatment may be contributing to this. Even transient azotemia affects long-term mortality [4,18,21,24]. This is why clinicians should understand and recognize AKI in the hospital setting. The association between AKI and higher mortality has been clearly demonstrated [1,2,3,4,5,6]. All previous consensus definitions and the majority of previous studies of AKI focused on the severity of AKI, as defined by the magnitude of serum creatinine elevation or the decrease in urine output. Numerous studies have confirmed that AKI severity does influence prognosis [10,11,12,13,14]. However, the duration of AKI has not been considered when assessing the severity of AKI. Recently, a few studies have suggested that duration of AKI is an additional predictive parameter influencing AKI severity [15,16,17,18]. Our results also support this. Previous prospective studies of patients undergoing cardiac and non-cardiac surgery confirmed earlier reports that transient and persistent AKI was associated with worse survival. Furthermore, they showed the duration of AKI after surgery was directly proportional to long-term mortality [15,16]. In critically ill patients, the duration of AKI was found to affect long-term mortality in a retrospective analysis [17]. However, the results of two large retrospective studies with hospitalized medical and surgical patients were not consistent [18,25]. Tian et al. [25] showed the mortality rate for patients with AKI that rapidly reversed was similar to the overall AKI cohort. In contrast, another study revealed that longer duration of AKI increased the risk of death [18]. However, the two studies used different definitions of AKI duration and only examined hospital mortality. In our study, using National Health Insurance Company data, we found that patients with longer duration (≥ 6 days) AKI had higher long-term mortality rates than those with shorter duration AKI. The NHIC covers all Koreans who are living in Korea. Mortality data from the NHIC is considered to be accurate.
The correlation between AKI duration and mortality in our study may be explained as follows. There was no difference in baseline characteristics between the two duration groups. However, in the longer duration AKI group, more patients were AKIN stage 3 (p = 0.003) and fewer recovered from AKI (p < 0.0001). AKIN stage 3 patients may be more likely to have multiple organ injuries and be more likely to receive a variety of medications that could affect the kidney. Han et al. [17] also found a correlation between AKI duration and renal recovery in their long-term follow-up study. Our analysis related to recovery from AKI also showed mortality as an effect.
Our study has some limitations. First, it is a single-center study with a relatively small cohort of patients. However, our data came from pure treatment-related HAAKI. Second, because in this study we did not consider urine output in AKIN staging, even though it is one evaluation criteria, we were not able to identify all HAAKI patients. However, a previous study demonstrated that serum creatinine determined the maximum AKIN stage achieved for 95% of patients, whereas in 5% of patients it was the urine output criteria that led to a maximum AKIN stage [26]. Third, the accuracy of our creatinine measurements has limitations. This is due to interference by some medications and bilirubin with our method of measuring creatinine, the Jaffe method [27]. Despite careful adjustments, we could not exclude the possibility of residual confounding factors. Finally, we defined AKI according to the AKIN criteria. A study comparing the AKI definitions showed that discrimination for hospital mortality was similar for the RIFLE and KDIGO criteria whereas the AKIN discrimination was inferior [28]. Thus, we may have underestimated HAAKI using the AKIN criteria.
The current classifications of AKI do not incorporate a duration component into their definitions. In our study, we demonstrated that AKI duration was associated with mortality. AKI duration provides additional valuable prognostic information for mortality. The incorporation of AKI duration into the consensus definitions of AKI classifications should be considered. Additionally, duration may be indicative of the potential for recovery from AKI. Thus, clinicians should quickly identify HAAKI and make an effort to shorten its duration to check reversible aggravating factors. Also, early detection of AKI using new biomarkers should be conducted. The utility of duration in assessing AKI should also be validated in larger, more varied cohorts.
KEY MESSAGE
The duration of hospital-acquired acute kidney injury is independently associated with longterm mortality.
Acknowledgments
This work was supported by the Soonchunhyang University Research Fund.
Notes
No potential conflict of interest relevant to this article was reported.