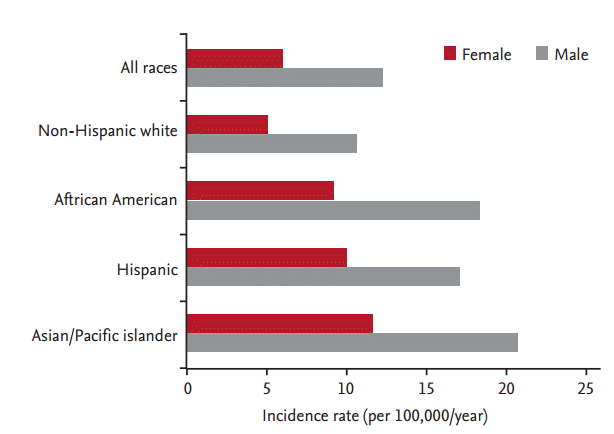Is screening and surveillance for early detection of gastric cancer needed in Korean Americans?
Article information
Abstract
The incidence rate of gastric cancer in Korean Americans is over five times higher than that in non-Hispanic whites, and is similar to the incidence of colorectal cancer in the overall United States population. In Korea, the National Cancer Screening Program recommends endoscopy or upper gastrointestinal series for people aged 40 years and older every 2 years. However, the benefit of gastric cancer screening in Korean Americans has not been evaluated. Based on epidemiologic studies, Korean Americans appear to have more similar gastric cancer risk factors to Koreans as opposed to Americans of European descent, though the risk of gastric cancer appears to decrease for subsequent generations. Therefore, in accordance with recent recommendations regarding screening for gastric cancer in Korea, endoscopic screening for gastric cancer in Korean Americans should be considered, especially in those with known atrophic gastritis/intestinal metaplasia or a family history of gastric cancer. In the future, additional studies will needed to assess whether a screening program for gastric cancer in Korean Americans will result in a survival benefit.
INTRODUCTION
The incidence of gastric cancer is estimated to be roughly 952,000 cases yearly, making it the fifth most common malignancy in the world, after lung, breast, colorectal and prostate [1,2]. More than 70% of cases occur in developing countries and half of all the worldwide cases occur in Eastern Asia [1]. The incidence of gastric cancer in the United States is low, especially in non-Hispanic White Americans (4.0 per 100,000 individuals) compared to Koreans (42.5 per 100,000 individuals) [3,4]. Furthermore, gastric cancer is the third leading cause of cancer death in both sexes worldwide. The highest estimated mortality rates are in Eastern Asia (24 per 100,000 in men and 9.8 per 100,000 in women), and the lowest in North America (2.8 per 100,000 in men and 1.5 per 100,000 in women) [1,2]. Asian Americans were the fastest growing immigrant group in the United States between 2000 and 2010 [5] and in 2011 totaled 18.2 million, with Korean Americans totaling 1.7 million.
Countries with high prevalences of gastric cancer have initiated screening programs, as early detection is associated with better outcomes [6]. In Korea, screening was initiated in 1999 [7] and involves upper endoscopy or upper gastrointestinal series (UGI) for patients 40 years or older every 2 years. As a result of this screening program, more than 50% of gastric cancers in Korea are diagnosed at an early stage [8], compared to fewer than 10% in Western countries [9]. Whether gastric cancer screening is beneficial in Koreans who have immigrated to the Unites States, and what those screening recommendations should be, remains unclear. According to a recent study surveying Koreans living in Washington State, roughly one-third of the responders had visited Korea for medical services within previous 5 years [10], further complicating potential screening recommendations. In this review article, we will address the potential benefits of gastric cancer screening and suggest a screening and surveillance strategy for Korean Americans.
IS GASTRIC CANCER SCREENING NEEDED IN KOREAN AMERICANS?
There is significant variability in the incidence of gastric cancer among different ethnicities in the United States. According to SEER (Surveillance, Epidemiology and End Results) data from 2002 to 2006, Asian Americans had the highest incidence rates, followed by African Americans and Hispanics (Asian men, 20.8 per 100,000/year; African men, 18.4 per 100,000/year; and Hispanic men, 17.1 per 100,000/year) (Fig. 1) [11]. Korean Americans have a significantly higher incidence rate (50.0 per 100,000 men and 26.3 per 100,000 women) and a higher mortality rate (31.5 per 100,000 men and 14.5 per 100,000 women) compared to other East-Asians (Japanese and Chinese) and Southeast Asians (e.g., Vietnamese) (Fig. 2) [12]. Furthermore, a recent study of cancer incidence and mortality in East-Asian Americans in California found that Chinese, Vietnamese, Korean, and Japanese Americans had higher rates of gastric cancer and death from gastric cancer compared to non-Hispanic European Americans, with Koreans having the greatest risk among these groups [13,14].
In addition, first generation immigrants have a higher incidence of gastric cancer between first generation and subsequent generations of Asian Americans. According to the effect of migration on gastric cancer incidence among Japanese in Hawaii, first generation Japanese Americans still had very high rates of gastric cancer [15]. However, rates declined and became similar to those of Americans of European descent after two generations. This observation suggests that environmental factors have a significant influence on the development of gastric cancer. However, this situation may not be directly translatable to the situation for Korean Americans since incidence rates appear to remain high for subsequent generations of Korean Americans. Therefore, there may still be a need for gastric cancer screening beyond the first generation of Korean Americans, especially those having additional risk factors for gastric cancer (discussed in the next section).
RISK FACTORS FOR GASTRIC CANCER
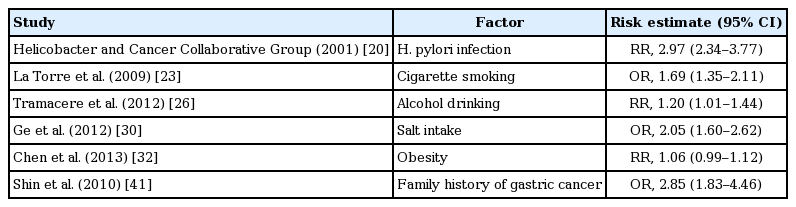
Gastric cancer is a multifactorial disease with both host and environmental factors playing a role in its etiology. Identified risk factors for gastric cancer include Helicobacter pylori infection, smoking, alcohol, obesity, salt intake, presence of atrophic gastritis (AG) and intestinal metaplasia (IM), and family history of gastric cancer. There are only limited available data on the status of gastric cancer risk factors in Korean Americans [16]. However, Korean Americans appear to have risk factors that are more similar to Koreans rather than Americans, which might explain the above-mentioned high gastric cancer prevalence in Korean Americans [17]. The relative risks (RRs) of gastric cancer with each risk factor are summarized in Table 1, and each risk factor will be discussed in the following sections.
Helicobacter pylori
H. pylori infection triggers a series of inflammatory reactions, which can subsequently lead to chronic gastritis. Progression from chronic gastritis to gastric atrophy and IM is an early step of mucosal changes in the stomach that can then lead to dysplasia and cancer [18]. H. pylori has been classified as group 1 carcinogen by the International Agency for Research on Cancer since 1994 because many studies have proven the association between H. pylori infection and development of gastric cancer, especially intestinal type non-cardiac gastric cancer [19]. A pooled analysis of 12 case control studies including 762 cases of non-cardiac gastric cancer showed that the RR of H. pylori on gastric cancer was 2.97 (95% confident interval [CI], 2.34 to 3.77) [20].
H. pylori organisms can be found in the stomach in about two-thirds of the world’s population, and H. pylori infection is more prevalent in countries with high gastric cancer incidence rates. There is wide discrepancy in the prevalence of H. pylori between Koreans and Americans. In Korea, the prevalence of H. pylori in adults was about 66.9% in 1998, which decreased to 59.6% in 2005 [21]. In the United States, H. pylori prevalence is variable among ethnicities (60% in Hispanics, 54% in African American, and 20% in Whites) [22]. The estimated overall prevalence is about 20% for people < 30 years and 50% for those > 60 years in the United States. Although a well-designed study for the prevalence of H. pylori in Korean Americans has not been published yet, Korea-born Korean Americans are thought to have a higher prevalence of H. pylori infection than United States-born Korean Americans.
Cigarette smoking
Smoking appears to be a moderate risk factor, compared with other tobacco-related cancers. A meta-analysis including 14,442 cases and 73,918 controls showed an odds ratio (OR) of 1.69 (95% CI, 1.35 to 2.11) for current smokers in comparison to those who have never smoked [23]. The risk for gastric cancer increases significantly with increasing amount and duration of smoking [24]. The effects of smoking may differ according to the location of gastric cancer; the RRs were 1.87 (95% CI, 1.31 to 2.67) for cardia cancers and 1.60 (95% CI, 1.41 to 1.80) for non-cardiac cancers in a meta-analysis based on nine cohort studies [25].
Alcohol
Drinking alcohol has been long suggested as a risk factor for gastric cancer. In a recent meta-analysis of 44 case control and 15 cohort studies including 34,500 cases of gastric cancer, the influence of light to moderate alcohol consumption results in a slight increase in risk, whereas heavy alcohol use (> 4 drinks per day) yields a more significant risk of 1.20 (95% CI, 1.01 to 1.44) [26]. However, in most prospective studies, the RR was not significantly elevated [27]. In a few studies that analyzed the risk for gastric cancer according to the tumor location, a slightly stronger association was found for non-cardia cancer than for cardia gastric cancer [26].
Salt intake
The daily sodium intake of Koreans is more than that of Americans (4,791 mg vs. 3,436 mg) [28]. The amount of daily salt intake is strongly related with the type of food consumed. Korean-Americans have a tendency to consume traditional Korean food (which has a high salt content, e.g., soy sauce, salt-preserved fish, and Korean style soup, etc.) even after immigrating to the United States. High dietary salt intake is known to be a risk factor for gastric cancer [29]. A meta-analysis including 11 studies (seven case controls and four cohorts) showed significant positive association between high salt intake and gastric cancer compared with low salt intake (OR, 2.05; 95% CI, 1.60 to 2.62) [30]. In another meta-analysis including only prospective population studies, high and moderately high salt intake were associated with increased risk of gastric cancer (RR, 1.68; 95% CI, 1.17 to 2.41; and RR, 1.41; 95% CI, 1.03 to 1.93, respectively) [31].
Obesity
Unlike esophageal adenocarcinomas in which obesity is a major risk factor, studies on the association between obesity and gastric cancer have shown conflicting results. A recent meta-analysis including 24 prospective studies found that obesity (body mass index [BMI] ≥ 30 kg/m2) was not associated with risk of gastric cancer (RR, 1.06; 95% CI, 0.99 to 1.12) [32]. In a pooled analysis of esophagogastric junction cancers, individuals with BMI of 25 to 30 kg/m2 had a 1.21-fold (95% CI, 1.03 to 1.42), and those with BMI of ≥ 30 kg/m2 had a 2.06-fold (95% CI, 1.63 to 2.61) increased risk of esophagogastric junctional cancer, including cardia gastric cancers, compared to individuals with BMI of < 25 kg/m2 [33]. In contrast, obesity is not a risk factor for non-cardia gastric cancer [34].
Atrophic gastritis and intestinal metaplasia
AG and IM have been regarded as pre-cancerous conditions of gastric cancer, and have a strong relation with H. pylori infection [35]. Therefore, AG and IM have been suggested as a useful clinical entity to identify those who are at higher risk for gastric cancer who would benefit from surveillance endoscopy. A nationwide cohort study in Netherlands reported that risk of gastric cancer increased in a step-wise manner correlating to the severity of premalignant gastric lesions (annual incidence of gastric cancer within 5 years after diagnosis: 0.1%, 0.25%, 0.6%, and 6% for AG, IM, mild-to-moderate dysplasia, and severe dysplasia, respectively) [36]. Although the risk of gastric cancer in individuals with AG differs according to the severity of AG, the adjusted RR of gastric cancer in the patients having severe AG was 5.76 [37].
IM is generally sub-classified as complete and incomplete type. Complete type (small intestinal type) IM expresses the full set of digestive enzymes, while incomplete type (colonic type) IM shows absent or incomplete expression. Incomplete type is considered to be the most advanced stage of IM and has higher risk of progressing on to gastric cancer [38]. A pooled analysis including 14 cross-sectional studies and 10 follow-up studies revealed that 13 out of 14 cross-sectional studies and 6 of 10 follow-up studies reported statistically significant higher gastric cancer prevalence in incomplete type IM than in complete type IM, and the RR of gastric cancer ranged from 4 to 11 [39]. Therefore, patients that have incomplete type IM should be considered for gastric cancer surveillance. Furthermore, H. pylori-infected individuals with IM were found to have a 6.5-fold higher risk of developing gastric cancer [40].
Family history of gastric cancer
Members of the same family tend to have shared genetic factors as well as similar environmental factors such as socioeconomic status and dietary habit. Therefore, caution should be used when interpreting the results of studies that report family history of gastric cancer as an independent risk factor of gastric cancer. A Korean study showed that adjusted OR for gastric cancer was 2.85 (95% CI, 1.83 to 4.46) for subjects with first-degree relatives with gastric cancer [41]. In addition, in a recent meta-analysis evaluating H. pylori infection and gastric histology in first-degree relatives of gastric cancer patients, family history of gastric cancer increases the risk of H. pylori infection, AG, and IM by approximately 2-fold each [42].
METHODS FOR SCREENING GASTRIC CANCER
Four methods have been used in gastric cancer screening: H. pylori serology, serum pepsinogen (PG) testing, UGI series, and endoscopy. The ideal screening test should be simple, safe, validated and cost-effective. Pros and cons of each screening method for gastric cancer are summarized in Table 2.
H. pylori serology
H. pylori serology itself is not an effective method of screening for gastric cancer because of low sensitivity and inability to detect premalignant lesions. In fact, it can be negative in the presence of longstanding AG and IM. Even though H. pylori virulence factors such as Cag A, Vac A, and Bab A may increase the sensitivity for detecting premalignant lesions, their sensitivity remains low [43]. Therefore, H. pylori serology alone is not useful as a screening test for gastric cancer.
Serum pepsinogen testing
PG is a precursor of pepsin produced in the gastric mucosa. Biochemically and immunologically, PG is classified into two different isozymes, namely PG I and PG II. PG I is produced by chief cells in the fundus and body, and PG II is produced throughout the entire stomach [44,45]. The serum PG level reflects not only the morphology and function of acid secretory glands but also pathological conditions of the gastric mucosa such as inflammation or atrophy [46-48]. A stepwise decrease in the serum PG I level and PG I/II ratio is closely associated with the progression of gastric atrophy [49,50]. Therefore, the serum PG level is considered to be a useful marker of AG, which is a precursor to intestinal type adenocarcinoma in the stomach.
To detect gastric cancer, the serum PG level, followed by endoscopic examination, was introduced in mass screening of high-risk patients for gastric cancer. In the case of PG I ≤ 70 ng/L and PG I/II ratio ≤ 3.0, the risk for gastric cancer increases. In fact, several prospective cohort studies reported that serum PG level is useful for evaluating the risk for gastric cancer [51-53]. A case-control study using serum PG level for gastric cancer screening showed the ORs for death from gastric cancer among control subjects screened within 1 and 2 years were 0.238 (95% CI, 0.061 to 0.929) and 0.375 (95% CI, 0.155 to 0.905), respectively [54]. In a pooled meta-analysis assessing approximately 300,000 people, the sensitivity and specificity of serum PG testing for gastric cancer screening were 77% and 73%, respectively [55]. Therefore, gastric cancer screening using serum PG level is suggested to decrease mortality from gastric cancer. However, it is unclear if this data is applicable to populations outside of Japan.
Upper gastrointestinal series
The barium meal indirect X-ray examination was introduced as a mass screening program of gastric cancer in the 1960s in Japan [56]. It is used widely in resident medical examinations under the National Cancer Screening for the aged, employer medical examinations, and personal medical examinations. Gastric cancer screening using UGI series suggests a reduction of mortality in a meta-analysis that included three Japanese case-control studies; however, the level of evidence is weak [57]. The sensitivity of UGI series ranged from 60% to 80%, whereas the specificity and true positive rates were 90% and 0.7% to 2.0%, respectively [48]. Most case-control studies conducted in Japan showed that screening by UGI resulted in a 40% to 60% reduction in gastric cancer mortality [58-60]. However, because of the lack of data from prospective series, that defined death from gastric cancer as an endpoint, UGI series receives a low grade recommendation for population-based gastric cancer screening [61].
Endoscopy
Although UGI series was the initial tool for mass screening for gastric cancer, a cohort analysis showed that the detection rate of early gastric cancer by endoscopy was approximately about 3- to 5-fold higher than that by UGI series and use of endoscopy was more cost-effective [62]. The sensitivity of endoscopy for gastric cancer screening was 78% to 84% [63,64]. As a result, endoscopy has become the main modality for gastric cancer screening in Korea and Japan. Although it has been suggested that endoscopic screening is cost-effective in high-incidence areas, there is no evidence that endoscopic screening is effective or cost-effective in average-risk populations [65]. Population-based endoscopic screening for gastric cancer has several limitations such as the need for experienced endoscopists, potential complication of endoscopy, and low acceptability by participants [48]. In addition, endoscopy may not be a practical strategy for mass screening in other countries where the cost of endoscopy is higher.
SCREENING PROGRAMS FOR GASTRIC CANCER AND THEIR EFFECTS IN KOREA
In Korea, the National Cancer Screening Program for gastric cancer screening was initiated in 1999 [7]. Endoscopy or UGI series is recommended for people aged 40 years and older every 2 years. In 2001 and 2003, the participation rate was only 11.4% and 13.6%, respectively [66]. Because of the government’s efforts to encourage cancer screening through media and by offering free screening, the rate of participation has been increasing [67]. The participation rate for gastric cancer screening in 2012 was 43.9% [68]. With increasing participation in gastric cancer screening, the number of patients diagnosed with gastric cancer through this screening program has increased, and has resulted in a higher proportion of early-stage cancer being diagnosed. This has resulted in an increased use of minimally invasive treatments such as endoscopic submucosal dissection [8]. As a result, approximately 46% to 67% of gastric cancers detected with endoscopy were early-stage cancers [69], and the 5-year survival rate has increased from 42.8% in 1993 to 1995, 46.6% in 1996 to 2000, 57.7% in 2001 to 2005, to 69.4% in 2006 to 2011 (Fig. 3) [4].
In a study comparing the performance of endoscopy with that of UGI series for gastric cancer screening, gastric cancer detection rates for endoscopy and UGI series were 2.61 per 1,000 and 0.68 per 1,000 screenings, respectively [70]. The sensitivity of endoscopy and UGI series screening to detect gastric cancer was 69.0% and 36.7%, respectively, and the specificity was 96.0% and 96.1%, respectively [69,70]. Recently, a study evaluating the cost-effectiveness of the National Gastric Cancer Screening Program demonstrated that the age-adjusted incremental cost-effective ratio for survival in endoscopy was 119,099,000 to 178,700,000 Korean won/survival, which was lower than that in UGI series (260,201,000 to 371,011,000 won/survival) [71]. In addition, it was determined that it would costs approximately 14,466,000 to 15,014,000 won per life-year saved for UGI series and 8,817,000 to 9,755,000 won per life-year saved for endoscopy. Furthermore, studies comparing endoscopy versus no screening demonstrated that endoscopy was the most cost-effective strategy [71-73]. Therefore, endoscopy is recommended as the first-line screening method in Korea.
SURVEILLANCE FOR GASTRIC CANCER
AG, IM, and dysplasia are considered precancerous lesions and require accurate surveillance programs. In a large western cohort study, the annual incidence of gastric cancer within 5 years was 0.1% for AG, 0.25% for IM, 0.6% for mild-to-moderate dysplasia, and 6% for severe dysplasia [36]. In another cohort study, the risk of progression to cancer within 10 years was 0.8% for AG, 1.8% for IM and 3.9% for low-grade dysplasia [74].
Currently, there are no international recommendations for surveillance of premalignant lesions. Recently, the International Consensus Project suggested the following recommendations for the management of patients with precancerous conditions [75]: (1) patients with mild-to-moderate atrophy and/or IM only in the antrum do not need follow-up (evidence level 4, recommendation grade D); (2) patients with extensive atrophy and/or extensive IM should be offered endoscopic surveillance (evidence level 2++, recommendation grade B) every 3 years (evidence level 4, recommendation grade D); (3) patients with low-grade dysplasia should be followed up every 12 months while those with high-grade dysplasia should be closely followed up every 6 months (evidence level 2+, recommendation grade C); and (4) patients with dysplasia or cancer within an endoscopically visible lesion should undergo staging and resection.
However, discrepancy between the results of endoscopic forceps biopsy and endoscopic resection is not uncommon [76,77], with approximately half of lesions interpreted as high-grade dysplasia on forceps biopsy being diagnosed as carcinoma after endoscopic resection [78]. Therefore, in cases with high-grade dysplasia, most clinicians agree that complete endoscopic resection or surgical removal of the tumor is necessary instead of close follow-up.
APPLYING SCREENING AND SURVEILLANCE PROGRAMS FOR GASTRIC CANCER TO KOREAN AMERICANS
It remains unclear who should be screened, when screening should be initiated, and how screening should be performed. It has been suggested that the screening strategy for gastric cancer should be based on incidence of the population and individual risk. The strategy of test and treat for H. pylori infection can be effective at reducing the incidence and mortality of gastric cancer in communities with a high incidence of gastric cancer. However, follow-up surveillance for gastric cancer is also necessary in patients requiring H. pylori eradication, because the risk of gastric cancer remains even after eradication of H. pylori. Therefore, the strategy of test and treat for H. pylori infection followed by evaluation with endoscopy to identify those who have developed AG or IM is needed in order to identify patients who are at higher risk of developing gastric cancer that should go on for surveillance.
In the United States, where gastric cancer incidence rates are low, a study that compared endoscopy with no screening showed that one-time screening for the general population at the age of 50 would cost $115,664 per quality-adjusted life year [79]. Furthermore, endoscopic screening of less advanced lesions was not cost-effective, except possibly for immigrants from high-risk Asian countries [80]. According to a guideline on ethnic issues in endoscopy by the American Society for Gastrointestinal Endoscopy [81], although screening for and treating H. pylori has the potential to reduce the risk for gastric cancer in groups with high gastric cancer risk, ethnicity based deviations from usual care is not suggested. In patients with gastric IM, surveillance is suggested for those at increased risk of gastric cancer due to ethnic background or a family history. Therefore, in accordance with recent recommendations regarding screening for gastric cancer in populations within Korea and Japan [82], endoscopic screening for gastric cancer in new (first generation) United States immigrants from high-risk regions around the world, such as Korea, Japan, and China, should be considered, especially if there is a family history of gastric cancer in a first-degree relative [81,83].
Although the evidence that Korean Americans have a high risk of gastric cancer similar to Koreans is clear, detailed data regarding the incidence according to generational status is lacking. In addition, differences in healthcare resources, screening strategy costs, and other healthcare-related factors exist. These differences require further investigation through more systematic, large scale studies. We have proposed a modified screening and surveillance program according to the presence of H. pylori infection with or without AG/IM or family history of gastric cancer in Korean Americans (Fig. 4). We recommend that Korean Americans undergo a screening EGD with biopsies (to determine H. pylori and AG/IM status) at the age of 40. Those at very low risk for gastric cancer (no H. pylori, no AG/IM) we suggest no further follow-up. Those at low risk (H. pylori infected, no AG/IM) should have H. pylori eradication therapy, and then undergo endoscopic examination every 3 to 5 years. In addition, H. pylori eradication should be confirmed. Those at high risk (presence of AG/IM, or family history of gastric cancer) should have endoscopy every 1 to 2 years with eradication therapy in cases with H. pylori infection. Those having low-grade dysplasia should have follow-up endoscopy in 6 to 12 months followed by annually endoscopic examination or undergo endoscopic resection if there is a visible lesion. Those having high-grade dysplasia should undergo endoscopic resection or follow-up endoscopy every 3 months. In the future, additional studies are needed to assess whether this screening program for gastric cancer in Korean Americans results in a survival benefit.
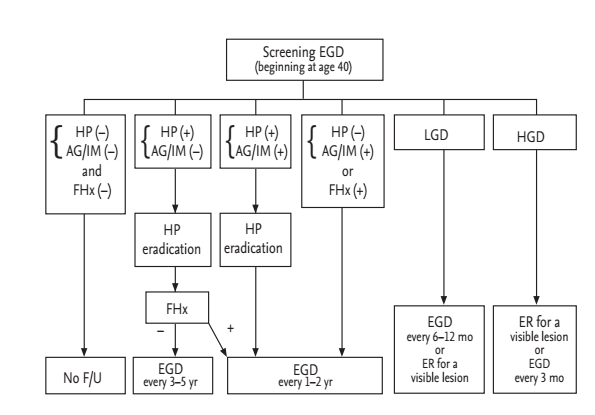
Suggested screening and surveillance program for gastric cancer in Korean American. EGD, esophagogastroduodenoscopy; HP, Helicobacter pylori; AG, atrophic gastritis; IM, intestinal metaplasia; F/Hx, family history of gastric cancer; LGD, low-grade dysplasia; HGD, high-grade dysplasia; F/U, follow-up; ER, endoscopic resection.
CONCLUSIONS
Based on epidemiologic studies, the incidence rate of gastric cancer in Korean Americans is over five times higher compared to non-Hispanic Whites. In addition, Korean Americans appear to have similar gastric cancer risk factors in terms of environmental exposures and genetic background as Koreans; although the risk of developing gastric cancer appears to decrease with subsequent generations. Therefore, in accordance with recent recommendations regarding screening for gastric cancer in Korea, endoscopic screening for gastric cancer in Korean Americans should be considered, especially in those with AG/IM or a family history of gastric cancer. However, to establish a systematic gastric cancer screening program in Korean Americans, there are several challenges such as identifying who should undergo screening, at what age should screening be initiated, and how should screening be performed. We have proposed an algorithm for gastric cancer screening in Korean Americans primarily based on data from the experience in Korea. In the future, prospective studies in a large population of Korean Americans will be needed to determine if gastric cancer screening is effective.
Notes
No potential conflict of interest relevant to this article was reported.