Risk factors and etiology of surgical site infection after radical neck dissection in patients with head and neck cancer
Article information
Abstract
Background/Aims:
Surgical site infection (SSI) is a major complication after radical neck dissection (RND) in patients with head and neck cancer (HNC). We investigated the incidence, risk factors, and etiology of SSI among patients who underwent RND.
Methods:
A retrospective cohort study was performed on HNC patients, excluding those with thyroid cancer, who underwent first RND at a teaching hospital between January 2006 and June 2010. Medical records were collected and analyzed to evaluate the risk factors and microbiological etiologies.
Results:
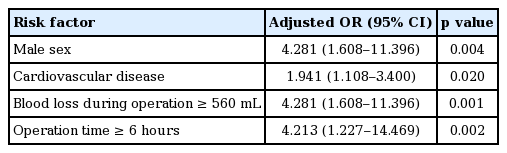
A total of 370 patients underwent first RND. The overall incidence of SSI was 19.7% (73/370). Multivariate analysis showed that male sex (odds ratio [OR], 4.281; p = 0.004), cardiovascular diseases (OR, 1.941; p = 0.020), large amount of blood loss during surgery (OR, 4.213; p = 0.001), and surgery lasting longer than 6 hours (OR, 4.213; p = 0.002) were significantly associated with SSI. The most common causative pathogen was Staphylococcus aureus (32.6%), and 93.2% of S. aureus isolates were methicillin-resistant. Klebsiella pneumoniae (13/92, 14.1%), Pseudomonas aeruginosa (11/92, 12.0%), and Enterococcus species (11/92, 12.0%) were also frequently detected.
Conclusions:
Based on our results, we predict that certain groups of patients are at high risk for SSIs after major HNC surgery. Preventive measures or close monitoring in these patients may be required to reduce the likelihood of postoperative SSIs. Furthermore, even though additional research is required, we would consider changing the prophylactic antibiotic regimens according to the causative organisms.
INTRODUCTION
Head and neck cancer (HNC) is the sixth most common type of cancer, accounting for an estimated 650,000 new cancer cases and 350,000 cancer deaths worldwide every year [1]. More recently, the incidence of oropharyngeal cancer in the younger population has been increasing [2]. Surgery is the preferred treatment for HNC despite the fact that treatment of HNC is complex and involves multiple modalities. Wide resection and reconstruction as standard therapies for HNC have improved cure rates [3]. In patients with HNC, surgical site infection (SSI) has been the most frequent and significant complication, at varying rates [4-7]. The development of an SSI can cause prolonged hospital stays, increased health care costs, and delayed access to postoperative adjuvant therapy [4,6,8]. Several studies have suggested numerous risk factors for SSIs, including underlying medical conditions, smoking, alcohol use, preoperative anesthesia risk (American Society of Anesthesiologists [ASA] score), body mass index (BMI), presurgical tracheostomy, previous surgery, length of preoperative hospital stay, prior chemotherapy or radiotherapy, and blood loss [4-17]. However, significant discrepancies exist between the findings of these studies, and independent risk factors remain unclear.
The current study was conducted to evaluate independent risk factors associated with SSIs involving the upper respiratory or oropharyngeal mucosa in HNC. In addition, we attempted to identify the causative organisms for these infections.
METHODS
Study design and patients
We performed a retrospective cohort study to evaluate risk factors for SSIs. We reviewed the electronic medical records of patients diagnosed with HNC from January 2006 to June 2010 at Hallym University Kangdong Sacred Heart Hospital, a 700-bed secondary-level university hospital at which the IIsong Head-and-Neck Cancer Center is located. Patients diagnosed with HNC underwent radical neck dissection involving the upper digestive or respiratory tract were included. In addition, only patients aged > 20 years who were undergoing their first operations were included. Patients undergoing thyroid gland surgery with or without lymph node dissection were excluded. All operations were performed by surgeons with more than 5 years of experience in head and neck major oncological surgery or reconstruction. All patients included in the study received prophylactic antibiotics, and all surgical sites were disinfected with povidone iodine before incision. All patients received routine postoperative care, and surgeons or infectious disease specialists diagnosed SSIs. The data collected included age, sex, history of smoking and alcohol consumption, underlying disease, primary site of tumor and TNM stage, ASA score, National Nosocomial Infections Surveillance System (NNIS) risk index, preoperative albumin level (mg/mL), BMI, blood loss during surgery (mL), blood transfusion during surgery, operation time, preoperative chemotherapy, preoperative radiotherapy, duration of hospitalization before operation, causative microorganisms, and prophylactic and therapeutic antibiotics. Severity of comorbid conditions was calculated using the Charlson comorbidity index [18]. This study was approved by the Institutional Review Board of Hallym University Kangdong Sacred Heart Hospital (Seoul, South Korea) as required by the local hospital policy at the time of the study.
Definitions
According to the Centers for Disease Control and Prevention’s NNIS and the criteria laid out by Horan et al. [19] and Johnson et al. [20], a SSI was defined by the occurrence of at least one of the following within 30 days of surgery: purulent drainage from the incision, spontaneous dehiscence or deliberate opening of the incisions with signs or symptoms of infection (pain, tenderness, localized swelling, redness, or heat), an isolated organism from the incision, purulent discharge from drainage, or an abscess without evidence of clinical anastomotic leakage. According to the guidelines, SSIs are classified as being either incisional or organ/space [21]. Incisional SSIs are further divided into those involving only skin and subcutaneous tissue (superficial incisional SSI) and those involving deeper soft tissue of the incision (deep incisional SSI). Organ/space SSIs involve any part of the anatomy other than incised body wall layers that were opened or manipulated during an operation.
Microbiologic methods
Microorganisms were identified by the VITEK 2 automated system (bioMerieux Inc., Hazelwood, MO, USA) in a microbiology laboratory. Antimicrobial susceptibility testing was performed using the GNI (gram-negative identification) card GPI (gram-positive identification) card in a VITEK 2 automated system according to the recommendations of the Clinical and Laboratory Standards Institute.
Statistical analysis
The chi-square or Fisher exact tests were used to compare categorical variables. Continuous variables were compared using the Student t test or Mann-Whitney test. To identify independent risk factors for infection, a stepwise forward logistic regression analysis was used to control for the effects of confounding variables. All risk factors with a p < 0.05 at the univariate level were included in the multivariate logistic regression.
All variables with a value of p < 0.05 were retained in the final model. Interactions between variables were not introduced into the models. Odds ratios and their 95% confidence intervals were calculated. All tests of significance were two-tailed, and those with a p value of < 0.05 were considered significant. SPSS version 18 (SPSS Inc., Chicago, IL, USA) was used for these analyses.
RESULTS
Clinical characteristics
During the study period, a total of 370 patients with HNC were included in this study. Out of the total of 370 patients who underwent major HNC surgery, 73 (19.7%) had SSIs. The vast majority of SSIs were in the form of deep incisional SSIs (40/73, 54.8%), followed by superficial incisional SSIs (18/73, 24.7%) and organ/space SSIs (15/73, 20.5%). The median time from surgery to diagnosis of SSI was 12 days (range, 1 to 29). Of the 73 SSIs, 52 SSIs (71.2%) developed within 14 days.
The mean age (± standard deviation) of the study population was 59.02 ± 13.09 years, and 290 patients (78.4%) were male (Table 1). The most common underlying disease was cardiovascular disease (35.4%, 131/370). The most common primary site of tumors was the hypopharynx (179 cases, 48.4%), followed by the oral cavity (114 cases, 30.8%), salivary gland (34 cases, 9.2%), nasopharynx/sinus (29 cases, 7.8%), neck (4 cases, 1.1%), and other (4 cases, 1.1%). The median duration of hospital stay was longer in patients with SSIs than in those without SSIs (78.0 [interquartile range (IQR), 40 to 119] vs. 41.0 [IQR, 24.0 to 87.5], p < 0.001). Table 1 shows the demographic characteristics of the patients and the incidence of SSIs. SSIs were found to be more frequent in men than in women (p = 0.001), and patients who were older (p = 0.019), smokers (p = 0.005), or consumers of alcohol (p = 0.041) were more likely to develop SSIs than their counterparts.
Risk factors for SSIs
The association of SSIs with preoperative variables is summarized in Table 1. Univariate analysis showed that cardiovascular disease (p = 0.006) and neurologic disease (p = 0.022) were associated with SSIs.
Table 2 summarizes the association of SSIs with perioperative variables. In the univariate analysis, an ASA score ≥ 3 (p = 0.005), NNIS risk index greater than 2 (p < 0.001), and operation time ≥ 6 hours (p = 0.007) were associated with SSIs. The amount of bleeding was greater in patients with SSIs than in those without SSIs (900 mL [IQR, 700 to 1,200] vs. 700 mL [IQR, 500 to 1,000], p < 0.001). We determined a cutoff point for developing SSIs. Using a receiver operating characteristic curve, we determined blood loss during the operation of more than 560 mL as the optimal cutoff point. Moreover, the amount of blood transfusion was higher in patients with SSIs than without SSIs, but this finding was without statistical significance (p = 0.051).
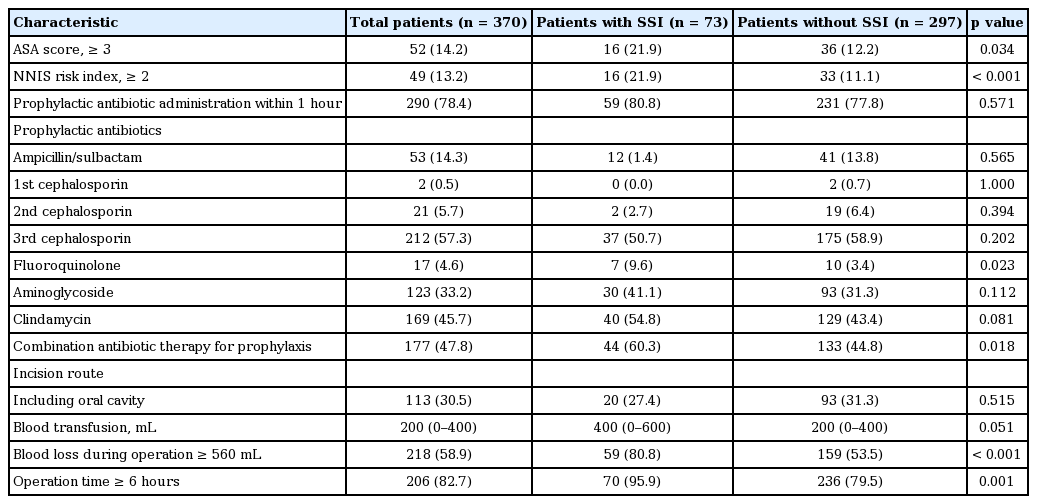
Perioperative variables associated with surgical site infections in patients undergoing radical neck dissection for head and neck cancer
Multivariate analysis revealed that being male, having a long operation time (over 6 hours), underlying cardiovascular disease, and blood loss during the operation of more than 560 mL were independent risk factors for SSIs (Table 3).
Causative microorganisms
A microbiological analysis was performed in all 73 patients with SSIs, and 60 patients showed positive culture results (Table 4). Twenty-nine patients had polymicrobial infections (29/60, 48.3%). A total of 92 isolates from 60 patients with SSIs included 47 gram-positive organisms (51.5%), 41 gram-negative organisms (43.5%), and 5 Candida species (5.4%). The most frequently isolated pathogen was Staphylococcus aureus (30/92, 32.6%), followed by Klebsiella pneumoniae (13/92, 14.1%), Pseudomonas aeruginosa (11/92, 12.0%), and Enterococcus species (11/92, 2.0%). Twenty-eight of the S. aureus isolates were methicillin-resistant S. aureus (MRSA) (93.3%). The resistance rates to cefotaxime and ciprofloxacin among the K. pneumoniae isolates were 30.8% (4/13) and 53.8% (7/13), respectively. The most common organisms associated with superficial incisional and deep incisional SSIs were S. aureus, followed by K. pneumoniae and P. aeruginosa. The distribution of pathogens showed a similar pattern in the two types of SSIs. S. aureus was the most common pathogen associated with organ/space SSIs, as expected. Enterobacter species were more commonly associated with organ/space SSIs (4/8, 50.0%) than with incisional SSIs.
Treatment outcomes of SSIs
Among the patients with SSIs, nine patients (12.33%) had bacteremia secondary to SSI. Most patients (82.2%, 60/73) improved within 7 days. However, 25 patients (34.3%) required surgical drainage, and one patient died as a result of complications of SSI. Vancomycin and piperacillin/tazobactam were widely used. Among the patients with SSIs, 29 patients (39.7%) received combination therapy with vancomycin plus piperacillin/tazobactam.
DISCUSSION
SSIs are one of the most common nosocomial infections that increase medical expenses [22]. Patients with SSIs tend to have increased morbidity and mortality. Major surgery for HNC frequently requires opening of the mouth floor, oropharynx, nasopharynx, or proximal esophagus, and these areas are likely to be contaminated by organisms residing in the mucosal surfaces. The 19.7% rate of SSIs observed in this study was consistent with the findings of another study published in South Korea [23] and previous studies [10]. However, our patients underwent surgery using clean-contaminated procedures with opening of the upper aerodigestive tract mucosa; therefore, the infection rate of our study is relatively lower than that observed in other studies under similar conditions [5,23]. Male sex was a significant risk factor for SSIs in the current study. This finding is consistent with the studies by Belusic-Gobic et al. [10] and Lee et al. [23], but inconsistent with other studies that reported an association between underlying systemic disease and infection [24,25]. In this study, among underlying systemic diseases, only cardiovascular disease showed a significant association with SSIs.
We also observed a significant correlation between long operation times and SSIs. If the operation time is prolonged, there are more chances for exposure to microorganisms in the mucous area; thus, allowing for more chances for infection.
In this study, the most common pathogen associated with SSIs was MRSA, followed by K. pneumoniae, P. aeruginosa, and Enterococcus species. Previous studies found similar results [5,10]. Moreover, 30.8% and 53.8% of K. pneumoniae isolates were resistant to cefotaxime and ciprofloxacin, respectively. According to common pathogens and antibiotic resistance rates, we could recommend a combination of vancomycin and anti-pseudomonal penicillin or anti-pseudomonal cephalosporin as initial empirical therapy.
All surgeries in this study were clean-contaminated and involved opening of the mucosa in the oral cavity or oropharynx or tracheobronchial tree. In a similar recent study by Lee et al. [26], early postoperative hypoalbuminemia was found to be the only independent risk factor for the development of SSI in patients undergoing major HNC surgery. Risk factors for developing SSI in colorectal surgeries known as clean-contaminated surgery were the presence of ostomy, preoperative steroids, and preoperative radiation [27]. Risk factors associated with SSI in patients undergoing clean surgery such as thyroidectomy were found to be obesity, alcohol use, and long operation time [28]. As mentioned above, the risk factors for SSI have been found to be different according to the type of surgery. In the current study, deep incisional SSI was the most common type of SSI, while superficial SSI was the most common type of SSI in clean surgery [28]. The incidence of deep incisional SSI and organ/space SSI in HNC surgery was higher than that in other types of surgery. Therefore, if SSI develops after HNC surgery, it may be necessary to conduct radiological examination early. In addition, clinicians should be aware of the implications of low postoperative albumin and consider more intensive postoperative care in HNC patients.
Our study has some limitations. First, because this study was of a retrospective nature, the possibility of a limitation in performing accurate comparisons should be borne in mind. This study was observational, and thus unknown risk factors might have been unequally distributed between the two groups. Second, the incidence of SSI could have been underestimated because mild cases may have been under-reported. Nevertheless, the chance of neglected data or cases is very low because all eligible patients were investigated and all patients received very careful postoperative follow-up.
In conclusion, 19.7% of our study population developed an SSI (73/370). Multivariate analysis showed that male sex, cardiovascular disease, blood loss during the operation of more than 560 mL, and a long operation time ≥ 6 hours were significant risk factors for SSI. Lastly, the most commonly discovered pathogen was MRSA. Based on our results, we are able to predict certain groups of patients who are at high risk of developing SSIs after major HNC surgery. Preventive measures or close monitoring in these patients may be required to reduce the likelihood of SSIs.
KEY MESSAGE
1. Of the 370 patients who underwent radical neck dissection, 73 (19.7%) developed surgical site infection.
2. Independent risk factors for surgical site infection were male sex, cardiovascular disease, blood loss during the operation of more than 560 mL, and a long operation time (≥ 6 hours).
3. The most common pathogen was methicillin-resistant Staphylococcus aureus.
Notes
No potential conflict of interest relevant to this article was reported.