Clinicopathologic significance of tumor microenvironment CD11c, and FOXP3 expression in diffuse large B-cell lymphoma patients receiving rituximab, cyclophosphamide, anthracycline, vincristine, and prednisone (R-CHOP) combination chemotherapy
Article information
Abstract
Background/Aims
CD11c is a dendritic cell marker in humans, which potentially induces a cytotoxic effect on lymphoma cells. Forkhead boxP3 (FOXP3) is a regulator of T lymphocyte in the microenvironment of the lymphoma. The principal objective of this study was to determine whether the tumors’ microenvironment expressions of CD11c and FOXP3 are predictive of clinical outcomes in diffuse large B-cell lymphoma (DLBCL) patients receiving treatment with rituximab, cyclophosphamide, anthracycline, vincristine, and prednisone (R-CHOP) combination chemotherapy.
Methods
The study population consisted of 100 patients with DLBCL. The CD11c and FOXP3 expression in primary tumors’ microenvironment were evaluated using an immunohistochemistry (IHC).
Results
CD11c and FOXP3 expression positivity in microenvironment were 25% and 35%, respectively. Each one counted for 1 point. In CD11c and FOXP3 stain, positive was counted as 0 and negative was 1. The points were separated into low risk (0 to 1) and high risk (2) groups. Only the extranodal DLBCL patient group analysis conveyed significant differences of progression-free survival (p = 0.019) and overall survival (p = 0.039) between the two groups.
Conclusions
We can achieve possible clinical significance of lymphoma tumor microenvironments through CD11c and FOXP3 IHC stains in extranodal DLBCL patients receiving R-CHOP therapy.
INTRODUCTION
Diffuse large B-cell lymphoma (DLBCL) is the most common type of lymphoma, accounting for approximately 40% of all non-Hodgkin lymphomas (NHLs) [1]. DLBCLs comprise a highly heterogeneous type with different clinical, morphological, immunological, and cytogenetic characteristics, treatment responses, and prognoses [2]. During the past decade, most studies on the heterogeneity of DLBCL have focused on genetic and molecular analyses. Gene expression profiling studies have identified two prognostically important subtypes of DLBCL; germinal center B cell (GCB)-like DLBCL and non-germinal center B cell (non-GCB)-like DLBCL [3,4].
Tumors are not just a mass of proliferating genetically abnormal cells, but are well defined as a heterogeneous and structurally complex tissue. Tumors contain a variety of cell types, including immune inflammatory cells, fibroblasts, and endothelial cells [5]. The assortment of cells and molecules collectively comprise the tumor microenvironment (TME). Emerging studies indicate that the TME is an important factor in the development and progression of cancer. The TME plays a dual role in cancer. It can not only suppress tumor growth by destroying cancer cells or inhibiting their outgrowth but also promote tumor progression either by selecting for tumor cells that are more fit to survive in an immunocompetent host or by establishing conditions that facilitate tumor outgrowth [6].
Regulatory T cell prevent self-destructive immune responses [7]. Recent evidence suggests that the cellular composition of the TME, presenting of tumor-infiltrating regulatory T cell, can significantly modify the clinical outcome in hematologic malignancies [8]. The transcription factor forkhead boxP3 (FOXP3) is uniquely expressed in regulatory T cell in the mouse and expression has been proposed as a lineage marker in developing regulatory T cells [9].
Dendritic cells (DCs) have a key role in the induction of adaptive immune responses [10]. DCs take up antigen, degrade them and express antigen peptides on their surface in the context of major histocompatibility molecules [11,12]. By this process, DCs become activated and involve up regulation of particular molecules on their surface that participate in T lymphocyte activation. The tumor infiltrating DCs are often not able to adequately stimulate T cells [13]. Several subsets of human DCs have been described, and significant differences in functional capacities of DC subsets were found with respect to changes in phenotypes, migratory capacity, cytokine secretion, T cell stimulation [14-16]. However, the exact functions of each DC subset and their consequences on the regulation of immune responses in vivo are still unknown. Human blood contains at least two distinct DC types, the myeloid DCs (mDCs) and the plasmacytoid DCs [17]. CD11c is often considered a marker for mDCs in humans, but it is also expressed by a subpopulation of human NK cells [18]. Thus CD11c is an important molecule in regulating immune responses. But, the relationship between mDCs and cancer prognosis is unclear.
The principal objective of this study was to determine whether the expressions of CD11c and FOXP3 in TME are predictive of clinical outcomes in DLBCL patients receiving treatment with rituximab, cyclophosphamide, anthracycline, vincristine, and prednisone (R-CHOP) combination chemotherapy.
METHODS
Patients
One hundred consecutive patients, who were diagnosed as DLBCL from December 2004 to May 2011, at Dong-A University Medical Center, Busan, Republic of Korea were included in the analysis. The criteria for case inclusion were the following: pathologically confirmed diagnosis of DLBCL suggested morphologic findings and immunophenotype suggested by 2008 World Health Organization classification [19] and availability of clinical data. The cases were re-reviewed by two expert hematopathologists whether the DLBCL is GCB or non-GCB depends on expression set of CD10, bcl-6, and MUM1 proteins by immunohistochemistry (IHC) suggested by Hans et al. [20]. The patients treated R-CHOP combination chemotherapy. The R-CHOP regimen was as follows: 375 mg/m2 rituximab, 750 mg/m2 cyclophosphamide, 50 mg/m2 anthracycline, and 1.4 mg/m2 vincristine were intravenously administered on day 1, and prednisone 100 mg was medicated on days 1 to 5. This regimen was repeated every 3 weeks. The following clinical data were collected from the record; patient demographics, Ann Arbor stage, international prognostic index (IPI), performance status, date of diagnosis, treatment response, date of relapse, date of last follow-up. This retrospective data collection of patients was approved by the Institutional Ethical Committee (DAUH-IRB-14-17).
Immunohistochemistry and assessment of immunostaining
Immunohistochemical study for the detection of CD10, bcl-6, and MUM1 expression was performed on core cancer tissues from each individual, which were arranged in tissue array blocks. The 4 to 5 μm sections were mounted on Superfrost Plus microscope slides (Thermo Scientific, Braunschweig, Germany) using the Benchmark XT automated IHC stainer (Ventana Medical Systems, Tucson, AZ, USA). Detection was performed with the Ventana Ultraview Universal DAB Detection Kit (Ventana Medical Systems). The slides were stained according to the following procedure. Tissue sections were deparaffinized using the EZ Prep solution (Ventana Medical Systems). For antigen retrieval, CC1 standard buffer (pH 8.4), containing Tris/Borate/EDTA (Ventana Medical Systems) was used for 60 minutes at 100°C. DAB inhibitor (3% H2O2 , Endogenous peroxidase; Ventana Medical Systems) was blocked for 4 minutes at a temperature of 37°C. The slides were incubated with primary antibodies: CD10 (Novocastra laboratories Ltd., Milton Keynes, UK [NCL-CD10-270]), bcl-6 (Cell marque, Rocklin, CA, USA [GI191E/A8]), MUM1 (Dako, Glostrup, Denmark [IS644]) for 32 minutes at 37°C, followed by incubation with an Univeral horseradish peroxidase (HRP) Multimer secondary antibody (Ventana Medical Systems) for 8 minutes at 37°C. The slides were incubated in DAB + H2O2 substrate (Ventana Medical Systems) for 8 minutes at 37°C, followed by hematoxylin and bluing agent counterstaining. Resection buffer (pH 7.6 Tris buffer; Ventana Medical Systems) was used as washing solution. According to CD10, bcl-6, and MUM1 expression, GCB or non-GCB DLBCL was divided by Hans’ criteria [20].
Immunohistochemical study for the detection of CD11c and FOXP3 expression was performed on core cancer tissues from each individual, which were arranged in tissue array blocks. The 4 to 5 μm sections were mounted on Superfrost Plus microscope slides (Thermo Scientific). Using the Discovery XT automated IHC stainer (Ventana Medical Systems), the slides were stained according to the following procedure. Tissue sections were deparaffinized using the EZ Prep solution (Ventana Medical Systems). For antigen retrieval, CC1 standard buffer (pH 8.4), containing Tris/Borate/EDTA, (Ventana Medical Systems) was used for 45 minutes. Inhibitor D of endogenous peroxidase (3% H2O2; Ventana Medical Systems) was applied for 4 minutes at a temperature of 37°C. The slides were incubated with primary antibodies: CD11c (Santa Cruz biotechnology Inc., Santa Cruz, CA, USA [sc-46676]), FOXP3 (Novus biologicals, Littleton, CO, USA [NBP1-43316)]) for 1 hour at 37°C, followed by incubation with an HRP-conjugated anti-rabbit/mouse secondary antibody for 16 minutes at 37°C. The reaction was detected with the Dako REAL Envision system (Dako). The slides were incubated in DAB + H2O2 substrate using the Ventana Chromo Map kit (Ventana Medical System) for 8 minutes at 37°C, followed by counterstaining with hematoxylin and bluing agent.
For the assessment of immunostaining, the intensity and distribution percentage of stained TME cells which is T cells or DC around tumor were evaluated. The slides were reviewed by an independent pathologist. The im munostaining were divided into negative and positive staining. The percentage scoring of the immunoreactive tumor cells was as follows: 0 (0%), 1 (1% to 10%), 2 (11% to 50%), and 3 (> 50%). The staining intensity was visually scored and stratified as follows: 0 (negative), 1 (weak, if it was a blush), and 2 (strong, if it was obviously positive at 20× magnification). A final score was obtained for each case by multiplying the percentage and the intensity score. Therefore, the tumors with multiplied score exceeding 4 (i.e., the tumors with a strong intensity of > 10% of the tumor cells) were recorded as having positive immunoreactivities for CD11c (Fig. 1B) and FOXP3 (Fig. 1D); all the other scores were considered as negative (Fig. 1A and 1C).

Immunohistochemistry f indings (A, B, anti-CD11c; C, D, anti-FOXP3, ×200). CD11c expression show (A) negativity and (B) positivity in intratumorally infiltrating mononuclear cells. Brown colored CD11c expresses at cell membrane. FOXP3 expression show (C) negativity and (D) positivity in intratumorally infiltrating mononuclear cells. Brown colored nuclear FOXP3 immunostaining is well recognized. FOXP3, Forkhead boxP3.
Statistical analysis
Overall survival (OS) and progression-free survival (PFS) were estimated using the Kaplan-Meier product-limit method. OS was measured from the data of treatment to the data of death or the last follow-up visit. PFS was calculated from the date of treatment to the first documented progression or death or the last follow-up visit. Survival rates were compared for statistical differences by using log-rank analyses.
RESULTS
Patients’ characteristics
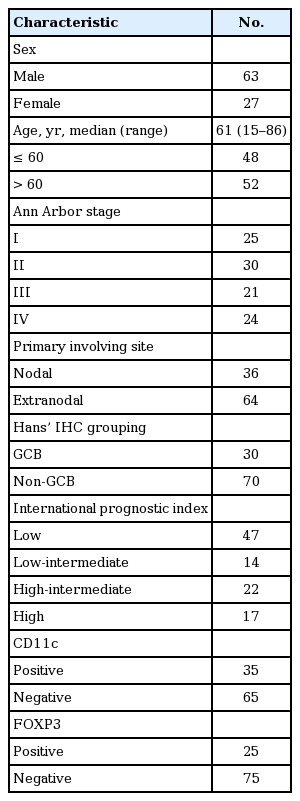
From December 2004 to May 2011, a total of 100 patents with positive histologic diagnosis from Dong-A Medical Center were enrolled. The clinical characteristics of the patients were shown in Table 1. Sixty-three patients were male and 37 were female. The median age of the patients was 61 years (range, 15 to 86). The Ann Arbor stages of the patients were I, II, III, and IV in 25, 30, 21, and 24, respectively. GCB and non-GCB of the patients was 30 and 70, respectively. Primary nodal and extranodal site involvements were 36% and 64%, respectively. According to the IPI, 47 cases were classified as low risk, 14 cases were classified as low-intermediate risk, 22 cases were classified as high-intermediated risk, and 17 cases were classified as high risk (Table 1).
Assay of immunohistochemistry
Non-tumoral mononuclear cells were represented by the membranous immunostaining of CD11c expression and the nuclear immunostaining of FOXP3 expression. CD11c (Fig. 1B) and FOXP3 expression (Fig. 1D) positive patients in microenvironment were 25% and 35%, respectively. Among CD11c expression positive patients, GCB and non-GCB was 12% and 23%, respectively. Among CD11c expression positive patients, primary nodal and extranodal DLBCL was 11% and 24%, respectively. Among FOXP3 expression positive patients, GCB and non-GCB was 10% and 15%, respectively. Among FOXP3 expression positive patients, primary nodal and extranodal DLBCL was 10% and 15%, respectively (Table 2).
Correlation with clinical outcome
During a median follow-up of 54 months, 37 patients had disease recurrence or death. The estimated 5-year PFS and OS were 57% and 65%, respectively (Fig. 2). There was no difference in PFS (p = 0.720) and OS (p = 0.552) according to GCB and non-GCB type. Also there was no difference in PFS (p = 0.861) and OS (p = 0.771) according to nodal and extranodal lymphoma.

Overall and progression-free survival curves of all patients. The estimate 5-year progression-free survival and overall survival were 57% and 65%, respectively.
The patients with CD11c expressing DLBCL had a significantly better PFS (p = 0.018) and OS (p = 0.05) rate than those without. While the patients with FOXP3 expressing DLBCL had no difference in PFS (p = 0.360) and OS (p = 0.457) rate than those without.
Extranodal DLBCL patient group analysis of OS and PFS was presented in Table 3. The patients with CD11c expression DLBCL had a significantly better PFS (p = 0.034) and OS (p = 0.058) rate than those without. The PFS (p = 0.246) and OS (p = 0.453) rate in patients with FOXP3 expressing DLBCL were not significantly better than those without.
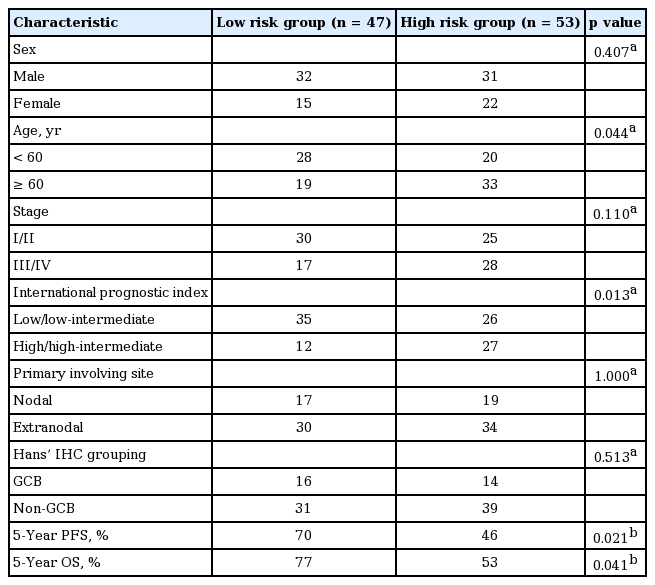
We classified patients according to the risk group. Each counted for 1 point; In CD 11c and FOXP3 stain, positive patient was counted as 0, negative was 1. The points were separated into low risk (0 to 1) and high risk (2) groups (Table 4). Older age over 60 and high IPI score were predominant in high risk group. Only extranodal DLBCL patients group analysis showed more significant differences of PFS (p = 0.016) and OS (p = 0.039) (Table 3, Fig. 3).
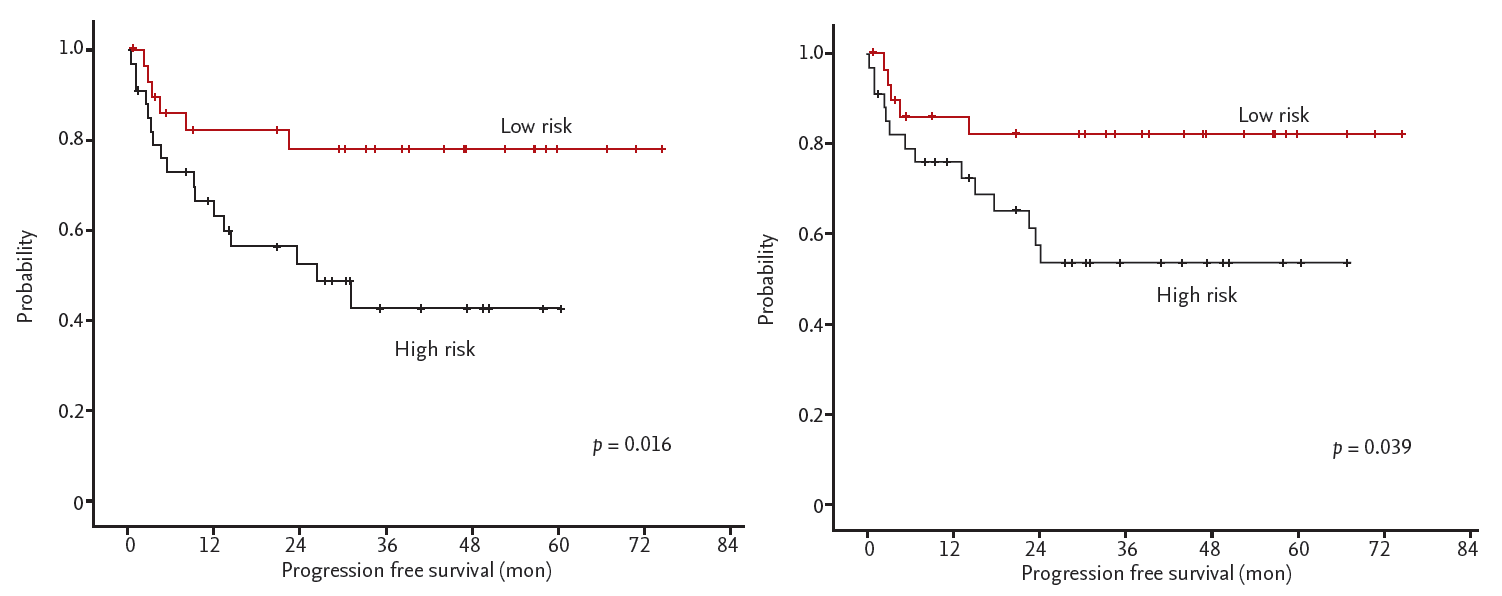
Survival curves of extranodal diffuse large B-cell lymphoma patients according to CD11c and Forkhead boxP3 (FOXP3). In CD11c and FOXP3 stain, positive was counted as 0 and negative was 1. The points were separated into low risk (0 to 1) and high risk (2) groups. It shows significant differences of (A) progression-free survival (p = 0.019) and (B) overall survival (p = 0.039) between the two groups.
In the multivariate analysis—including age, gender, nodal/extranodal involvement, GCB/non-GCB, stage, IPI, and risk group of microenvironment—for PFS and OS, IPI was prognostic factor for PFS (p = 0.010) and OS (p = 0.006).
DISCUSSION
For more than a decade, the clinical factors described by the IPI have been used for risk stratification of patients with DLBCL. However, DLBCL patients from identical IPI risk groups show considerably variable outcomes, indicating the biological and clinical heterogeneity of this disease [4,21]. In recent years, more attention has been paid to the biological heterogeneity of DLBCL. Currently DLBCL can also be classified into two subtypes based on microarray assay: GCB and non-GCB, which have different clinical characteristics and prognoses.
Using DNA microarrays, the two DLBCL subgroups are distinguished by the differential expression of hundreds of different genes, and these genes relate each subgroup to a separate stage of B-cell differentiation and activation. One type expresses genes characteristic of GCB, the other type expresses genes normally induced during in vitro activation of peripheral blood B cells (APB). Patients with GCB-like DLBCL had a significantly better OS than those with APB-like DLBCL [22]. However, the clinical application of molecular subtype classification is limited because gene expression profiling is time-consuming, expensive, and involves a complex analysis. Using the results of IHC, the submitted cases were classified into GCB or non-GCB subtypes based on the previously established algorithms (Hans, Muris, Choi, and Tally algorithms). Consistent with previous reports, we found that the GCB DLBCL was less frequent in Asian patients than in white patients. For example, 29% of Japanese patients had the GCB DLBCL whereas 71% had the non-GCB DLBCL [23]. Similarly, our study indicated that there were more GCB DLBCL than non-GCB DLBCL patients. But the subgrouping did not correlate with prognosis [24]. Our study also showed that there was no difference in GCB and non-GCB type.
The primary site of the lymphoma, either the lymph node or different extranodal territories, can separate two different groups of DLBCLs with particular clinicopathological features and different natural history [25]. Genetic differences between nodal and extranodal DLBCLs might exist, including single gene alterations, such as c-MYC, BCL-6, REL, and FAS (more frequently seen in extranodal DLBCL) [26]. Extranodal DLBCL is associated with older age and poorer performance score, but also lower tumor burden [27]. Performance status, IPI, B symptoms, and serum β2-microglobulin are prognostic factors in DLBCL patients. These prognostic factors usually affect both nodal and extranodal DLBCL. However, unlike nodal disease, primary extranodal DLBCL has a separate genetic origin [28-30].
Regulatory T cell, which was first named by Sakaguchi et al. [31], has become a principal focus of immunological studies over the past 20 years. FOXP3 is a transcription factor belonging to the forkhead family. Forkhead transcription factors perform an important function in cell proliferation, differentiation, and development [32]. In our study, we utilized FOXP3-immunohistochemical staining as a method for the detection of tumor infiltrating regulatory T cell. The FOXP3 cell density varies between different lymphoma types. Infiltrated FOXP3 cells in lymphoma microenvironments may represent important lymphoma/host microenvironment-modulators since increased amounts of these cells can positively influence survival in DLBCL. The increased percentage of FOXP3-positive regulatory T cell is predictive of improving OS in DLBCL [33,34], independent of the IPI. A better understanding of the biologic role of FOXP3-positive regulatory T cell in these tumors should assist in the development of therapeutic strategies based on the immunomodulation of the TME.
CD11c is often considered a marker for mDCs in humans, but it is also expressed by a subpopulation of human NK cells [35]. DCs and NK cells develop from a common intermediate progenitor.
In our study, all the 100 patients with DLBCL received standard R-CHOP regimen. We assessed the IPI score, origin—extranodal and nodal—and subgroups, including GCB and non-GCB, in a comparative study on the prognostic impact of tumor infiltrating FOXP3-regulatory T cell and CD11c positive DC. The results of previous studies showed the tumor infiltrating FOXP3 cells in lymphomas by taking advantage of lymphoma TME [33]. High expression FOXP3 DLBCL patients had better outcome than FOXP3 negative DLBCL patients [34]. In our study, TME was more relevant in extranodal than nodal DLBCL. Extranodal DLBCL group analysis showed more significant differences in PFS and OS. CD11c and FOXP3 expression was predictive of clinical outcomes in extranodal DLBCL patients. There has been a discussions about differences of biologic and clinical features between nodal and extranodal DLBCL [25]. It is unclear that what makes this clinical outcome difference between nodal and extranodal DLBCL. Tumor factors (GCB/non-GCB) and clinical factors (stage, IPI, age) could be causes of it. Also microenvironment of tumor cells could be a reason. It is hard to define the level of affection of microenvironment. But some differences and changes of microenvironment among anatomical differences or nodal/extranodal sites in B-cell NHL might be exist [36,37]. Through our data, we could guess possibility of different microenvironment effect between nodal and extranodal DLBCL.
The limitations of our study are its retrospective character, small sample size, and a single center study. Prospective large scale study is needed to confirm our study results. Furthermore, multi-center study is needed. We were unable to elucidate the clinicopathophysiology of DLBCL. The role of TME in cancer cells, regulatory T cells, and DCs on lymphoma is unknown. Retrospective study indicates that regulatory T cell and DCs mediate anti-tumor factor production. Further studies will be required in order to understand the basic antitumor mechanisms of FOXP3-positive regulatory T cell and CD11c-positive DC in hematological malignancies, including DLBCL.
KEY MESSAGE
1. It could be guessed possibility of different microenvironment effect between nodal and extranodal diffuse large B-cell lymphoma (DLBCL).
2. CD11c and FOXP3 expression was predictive markers of clinical outcomes especially in extranodal DLBCL patients treated with rituximab, cyclophosphamide, anthracycline, vincristine, and prednisone (R-CHOP).
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was supported by the Pioneer Research Center Program through the National Research Foundation of Korea funded by the Ministry of Science, ICT & Future Planning (2012-0009583 and 2012-0009664).