Chronic lymphocytic leukemia: a clinical review including Korean cohorts
Article information
Abstract
Only 5th decade ago, chronic lymphocytic leukemia (CLL) was only recognized as disease group of presenting features like peripheral lymphocytosis, organomegaly including of splenomegaly. As understanding of disease biology and molecular diagnostic tools are getting improved gradually, characterization of variation in CLL’s clinical courses was facilitated, resulting in better risk stratification and targeted treatments. Consequently multiple new targeted agents have been used in treatment of CLL, it makes improved clinical outcome. Rituximab containing chemoimmunotherapy (combination of rituximab, fludarabine, and cyclophosphamide) have shown better overall response rate and progression-free survival on fit patients’ group in front-line setting, result in standard first-line therapeutic option for CLL. Furthermore, after introducing that the B-cell receptor is crucial for the evolution and progression of CLL, emerging treatments targeting highly activated surface antigens and oncogenic signaling pathways have been associated with several successes in recent decades. These include new anti-CD 20 monoclonal antibody (obinutuzumab), the bruton tyrosine kinase inhibitor (ibrutinib), the phosphatidylinositol 3-kinase inhibitor (idelalisib), and B-cell CLL/lymphoma 2 inhibitor (ABT-199 and ABT-263). So, we discuss not only general pathophysiology of CLL, but also rapidly advancing treatment strategies that are being studied or approved for treatment of CLL.
INTRODUCTION
Chronic lymphocytic leukemia (CLL) is common hematologic disease in Western societies with over 15,000 patients were diagnosed annually [1], but is very rare in Asian cohorts as well as Korea. The molecular diagnostic tools and variable understanding of the pathobiology of the disease have facilitated characterization of variations in the disease’s clinical course, resulting in better risk stratification [2]. Consequently more precise targeting of therapy is possible, and the rapid evolution of treatment strategies for CLL and development of new drugs warrant a closer look into CLL. In consideration that the course of CLL typically has medical problems related to chronic infection, immunosuppressive status, autoimmune complication and surveillance of secondary malignancies, long-term relationship between medical practitioners and patients is need to solve those problems. Also, in Korea, the incidence of CLL has been gradually increased too and it is not the orphan disease any more in recent years, so we have plan to describe the generalized review about CLL, to intend to bring attention to take a profound interest in the field of CLL for the Korean medical doctors and researchers.
CELLULAR ORIGIN OF CHRONIC LYMPHOCYTIC LEUKEMIA
There are significant geographical variations in the incidence of CLL. CLL is the most common adult B-cell leukemia of elderly patients in the Western world, but is less common among people of African or Asian origin [1]. Although the exact cellular origin of CLL remains a matter of controversy [2], approximately half of all CLL cases have unmutated IgV genes (uCLL). The remaining cases carry somatically mutated IgV genes (mCLL) [3]. This distinction is of biological interest and clinical relevance because uCLL is more aggressive, with a significantly shorter time from diagnosis to initial treatment [4].
Identification of the cellular origin of CLL is essential to elucidating the pathobiology of a tumor. Only then can the full natural course of the disease be revealed. It is also important to understand the dysregulation of gene expression and cellular functions [5]. The cellular origin of CLL as uCLL and mCLL is delineated by the absence or presence of IgV genes, respectively, from memory B-cells in a post-germinal center. However, based on a recent report on specific IgV gene rearrangements, a derivative of uCLL (CD5+, CD27-CD38low) from conventional naive B-cells was proposed. mCLL (CD5+CD27+IgM+B-cell lymphoma [Bcl] 6mutIgVHmut) derives from a distinct, previously unrecognized B-cell subset from postgerminal centers [6].
INCIDENCE OF CHRONIC LYMPHOCYTIC LEUKEMIA IN KOREA
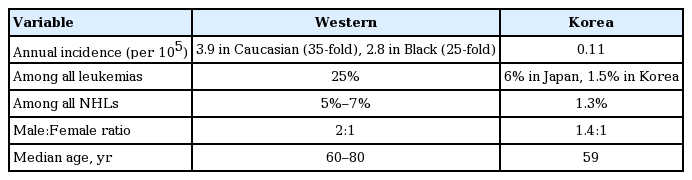
According to the crude incidence rate of non-Hodgkin lymphoma and the ratio of CLL in lymphoma, the crude incidence rate per 100,000 individuals was 0.11. In Korea, 56 patients are newly diagnosed with CLL annually. The crude prevalence rate of CLL is 0.61 per 100,000. In total, 306 patients had prevalent CLL from the National Cancer Incidence Database (KNCI DB) in Korea (Table 1) [7]. The population of Korean patients have been limited, but according to a few reported data of single center, Korean patients diagnosed with CLL have more atypical immunophenotype with high frequencies of FMC7+ and strong presentation with CD22+ and more aggressive than that in Western populations [8,9]. And also considering an increasing incidence rate, and improvements in treatment, the incidence and prevalence rates are expected to be relatively high.
CYTOGENETIC ABNORMALITY OF CHRONIC LYMPHOCYTIC LEUKEMIA
Conventional metaphase cytogenetic techniques are difficult in CLL due to the very low proliferative activity of leukemia cells in vitro. Therefore, interphase cytogenetic analysis with fluorescence in situ hybridization (FISH) is the standard method to detect chromosomal abnormalities that may have prognostic significance. Cytogenetic abnormalities that can be detected by FISH are present in approximately 80% of patients with previously untreated CLL. In a German study, the most common abnormality was del(13q) (55%), followed by del(11q) (18%), trisomy 12 (16%), and del(17p) (7%) [10]. In the 2014 National Comprehensive Cancer Network guidelines, CLL was classified into three risk groups by FISH, with del(17p) as TP53 gene found to be the strongest predictor for poor prognosis (Tables 2 and 3) [9,11-18]. In contrast with the results of Western societies, trisomy 12 was more common abnormality, followed by del(13q14), del(11q22), and del(17p13) in Korean CLL data [9].
CLINICAL MANIFESTATIONS AND DIAGNOSIS
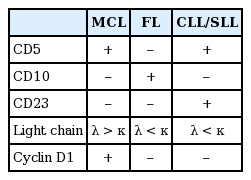
CLL is usually asymptomatic and found during a routine blood test. However, some patients consult a physician because of painless swelling of the lymph nodes, often in the cervical area, which spontaneously enlarge and shrink but do not disappear. CLL is usually first suspected through the presence of lymphocytosis. According to the 1996 International Workshop on CLL update of the National Cancer Institute guidelines for CLL diagnosis and treatment, an absolute B lymphocyte count in the peripheral blood of ≥ 5,000/µL (5 × 109/L) sustained for longer than 3 months must be observed [19]. In the current system, patients with an absolute lymphocyte count less than 5,000/µL (5 × 109/L) are usually diagnosed with monoclonal B-cell lymphocytosis rather than CLL if they do not have palpably enlarged lymph nodes, spleen, and/or liver [20]. Immunophenotyping, usually by flow cytometry, is a key component to the diagnosis of CLL [21]. Major immunophenotypic analyses of CLL show the expression of B cell-associated antigens, including CD19, CD20 (usually weak), and CD23, with characteristic expression of CD5, a common T-cell antigen [19]. In addition, the κ or λ light chain restriction pattern and surface IgM and IgD expressions are decreased. Immunohistochemistry of lymph node biopsies show that they were positive for CD5, CD20, and CD23, and negative for CD3, CD10, and cyclin D1 (Table 4). Because of CD5 expression in CLL, mantle cell lymphoma may mimic CLL. However, neoplastic cells from mantle cell lymphoma stain strongly for cyclin D1 and surface membrane immunoglobulin, have a t(11;14) chromosomal abnormality, and are CD23-negative. In contrast, malignant cells in CLL are cyclin D1-negative and often CD23-positive [22].
CLINICAL STAGING
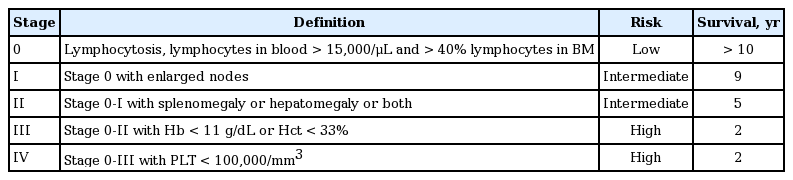
The two staging systems for CLL are the Binet staging system, proposed in 1981 (Table 5) [23], and the Rai staging system, suggested in 1975 (Table 6) [24]. The Binet staging system is used more frequently.
PROGNOSTIC FACTORS FOR CHRONIC LYMPHOCYTIC LEUKEMIA
CLL patients in the high-risk group include those who are refractory to fludarabine chemotherapy, who fail to achieve a response or show disease progression within 24 months of rituximab-based combination chemotherapy, or who have a TP53 mutation or del(17p). These high-risk patients have less than 36 months of overall survival after chemotherapy. We routinely performed FISH to detect chromosomal aberrations that are strong poor prognostic markers (Table 7). The identification of new genetic lesions in CLL has prompted the development of a comprehensive and dynamic prognostic algorithm. This algorithm considers gene mutations, chromosomal abnormalities, and their changes during recent clonal evolution (Table 8) [25]. However, this model requires both additional verification and a solution to its high cost.
TREATMENT OPTIONS
CLL is an extremely heterogeneous disease and most patients have early stage disease at the time of diagnosis. Therapy is indicated for patients with “active disease,” as manifested by an advanced stage, high tumor burden, disease-related “B” symptoms, or repeated infections [26,27].
Previously untreated patients
The selection of therapeutic options for previously untreated patients should consider the patient’s age, comorbidities, performance status, and genetic testing, especially for del(17p). In CLL without comorbidities or del(17p), the standard regimen is rituximab, fludarabine, and cyclophosphamide (R-FC). The R-FC regimen has higher rates of overall survival, complete response (70%), longer median progression-free survival, excellent induction effect, and minimal residual disease [28-30]. However, neutropenia and infusion-related toxicities were more common in patients receiving this regimen [31]. In elderly patients (> 70 years), those with poor performance status, or unavailable hospitalization, a combination regimen of rituximab and chlorambucil is the best option for first-line treatment [32]. Unfortunately, in Korea, only chlorambucil single method is available by Korean Health Insurance Review & Assessment Service. In high risk patients with del(17p) or TP53 mutations, alemtuzumab therapy followed by allogeneic stem cell transplantation is recommended (Table 9) [33,34].
Relapsed or refractory patients
For patients relapsing after or refractory to first-line therapy, treatment options depend on the duration of the response following first-line treatment [35]. In CLL patients with a time to treatment failure of 2 years or more, retreatment with the same regimen used as first-line therapy is recommended. However, in patients with relapsed disease within 2 years of the initial therapy, the suggested treatment options are alemtuzumab monotherapy, a combination of rituximab and bendamustine, other clinical trials (ibrutinib, idelalisib, and ABT-199), or allogeneic stem cell transplantation (Table 9).
New drugs
CLL is the most common leukemia in adults in Western countries, accounting for approximately 30% of all leukemia in the United States [36]. A broad range of novel agents with different mechanisms of action are being evaluated in clinical trials, with some already showing both efficacy and a favorable safety profile. These include combinations of agents already used in CLL therapy, agents approved for other diseases (such as lenalidomide and dasatinib), new antibodies (such as anti-CD37 antibodies), and other novel agents (such as flavopiridol, bruton tyrosine kinase (BTK) inhibitors other than ibrutinib, phosphatidylinositol 3-kinase inhibitors other than idelalisib, and Bcl-2 antagonists [37-39].
New drugs for CLL are divided into two groups: one group targets the B cell receptor (BCR) signaling pathway and the other, leukemia cell-specific molecular markers. Essential components of BCR signaling pathway include Lyn, Syk, and BTK. Ibrutinib (PCI-32765) as BTK inhibitor was associated with a high frequency of durable remissions (overall survival, 88%) in patients with relapsed or refractory CLL and small lymphocytic lymphoma. This included patients with high-risk genetic lesions (28 patients; overall survival, 68%) [40]. A combination of idelalisib and rituximab, compared with placebo or rituximab alone, significantly improved progression-free survival, response rate, and overall survival among patients with relapsed CLL who are less suitable for chemotherapy [41]. ABT-199, a selective Bcl-2 blocker that neutralizes Bcl-2 with high selectivity over Bcl-XL, also shows good response without thrombocytopenia, unlike navitoclax, which causes severe thrombocytopenia [42]. The representative new drugs mentioned above, ibrutinib, idelalisib, and ABT-199, are oral agents. More amounts of promising clinical data are expected in the future. A combination of an anti-CD20 antibody with chemotherapy improved outcomes in patients with CLL and comorbidities. In this patient population, obinutuzumab was superior to rituximab when in combination with chlorambucil (Fig. 1) [43,44].

Cellular markers and new drugs in chronic lymphocytic leukemia (CLL). A large number of cellular biomarkers have been found to correlate with prognosis in patients. Biomarkers can be grouped into the following functional categories. First, genetic lesions like loss of function of the DNA-damage response by TP53 or ATM (ataxia telangiectasia mutated) (i.e., del(17p), del(11q)), especially with respect to DNA-damaging chemotherapy like f ludarabine. Second, epigenetic modifications, for example, DNA methylation of CpG dinucleotides in the ZAP-70 (ζ-chain-associated protein kinase 70) gene as a surrogate marker for the immunoglobulin heavy-chain variable region (IGHV) hypermutation status. Third, surface markers like CD49d that correlate not only with prognosis, but also with genetic aberrations like trisomy 12 are possibly involved in mobilization and homing of CLL cells. Fourth, levels of microRNA genes like miR34a as readout for activity of TP53. Soluble serum markers of prognostic relevance such as levels of thymidine kinase and β2-microglobulin are not shown. With the advent of novel therapeutic compounds targeting bruton tyrosine kinase (BTK) or phosphatidylinositol 3-kinase (PI3K), the significance of current biomarkers will have to be re-evaluated. Adapted from Mertens et al. [44], with permission from American Society of Clinical Oncology. BCR, B cell receptor; Bcl-2, B-cell lymphoma 2; CXCR4, chemokine (C-X-C motif) receptor 4; VCAM-1, vascular cell adhesion molecule 1; SDF1, stromal cell-derived factor 1.
Allogeneic hematopoietic stem cell transplantation
Patients with CLL have the general characteristics with old age-onset and the relatively benign course of the disease in the majority of patients. So, only a selected subset is considered for aggressive treatments such as hematopoietic stem cell transplantation (HSCT). CLL transplantation criteria include a non-response or relapse within 12 months after purine analog-containing therapy, relapse within 24 months after purine-analog combination therapy or similarly efficacious therapy (autologous stem cell transplantation), and del(17) or TP53 deletion/mutations requiring therapy [45].
Cell therapy
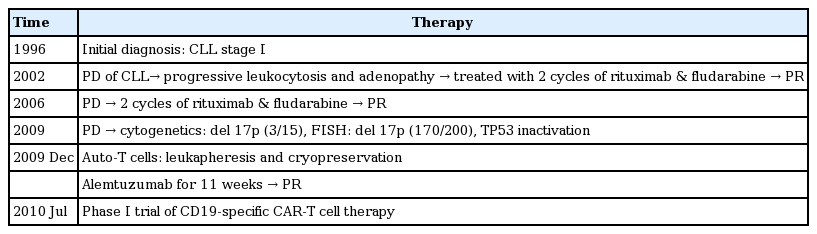
A pilot clinical trial in patients with relapsed/refractory p53-deficient CLL was performed using CD19-specific chimeric antigen receptor T-cell therapy [46]. This therapy regimen is strongly expected to provide an alternative treatment option in CLL patients who are refractory to conventional treatment (Table 10).
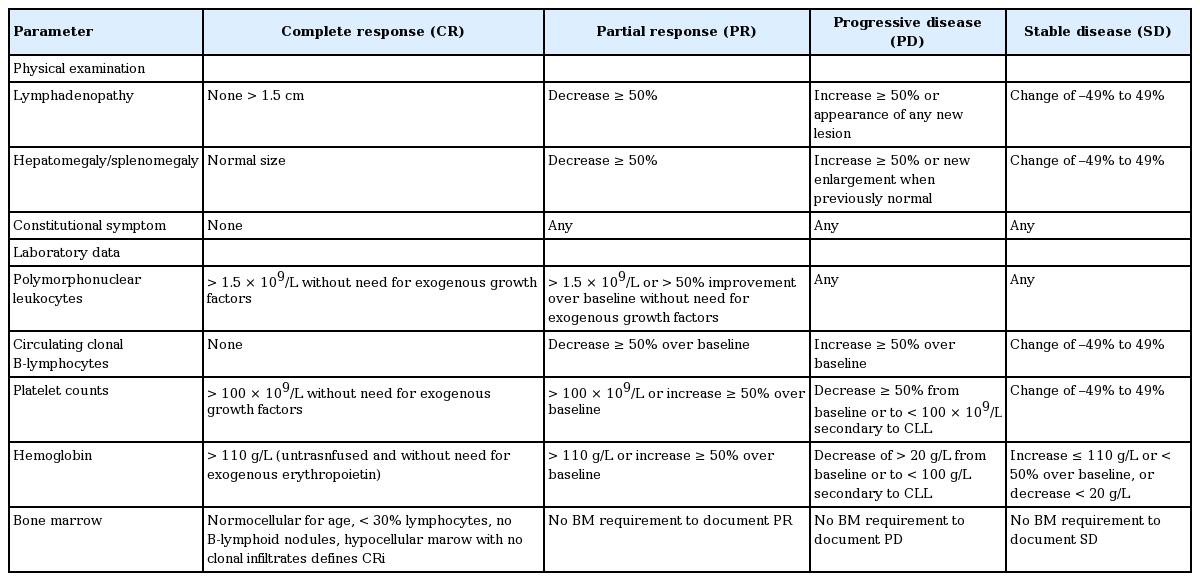
Tumor response assessment
Tumor response assessments for CLL are distinctive compared to other lymphoma subtypes. Response assessment involves both tumor burden and evaluation of hematopoietic function. For complete remission, all of the following criteria must be met without cancer related-constitutional symptoms. For partial response, at least two of the following group A criteria must be met over at least 2 months and at least one of the group B criteria must be met (Table 11) [19].
RARE COMPLICATIONS OF CHRONIC LYMPHOCYTIC LEUKEMIA
Infectious complications
Patients with CLL are susceptible to infectious events due to a reduction in immunoglobulin levels, decreased function of T-cells, other lymphocytes, and monocytes. Administration of immunoglobulin, prophylactic bactrim, and vaccination are necessary to minimize infectious events [47].
Richter syndrome
Approximately 2% to 10% of patients with CLL will develop Richter syndrome during transformation to diffuse large B-cell lymphoma or Hodgkin lymphoma during the course of the disease [48]. The cumulative incidence increases by approximately 0.1% per year. Diagnostic clues include increased serum lactate dehydrogenase, B symptoms, and rapidly enlarged lymph nodes. Biopsy confirmation is needed, and positron emission tomography-computed tomography is helpful in selecting the target for biopsy. The median time to development of Richter syndrome after CLL diagnosis is 1.8 to 5 years [49]. Prognosis is poor, with an overall survival of 5 to 8 months from initial diagnosis [50]. Patients with Richter syndrome should be treated with combination chemoimmunotherapy, such as R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone), HyperCVAD (cyclophosphamide, vincristine, doxorubicin, and dexamethasone), and OFAR (oxaliplatin, fludarabine, cytarabine, and rituximab) regimens [51]. Early allogeneic HSCT has shown promising results with Richter syndrome in younger patients [51].
Autoimmune cytopenias
Autoimmune hemolytic anemia (AIHA) is the most common form of autoimmune cytopenia, with an incidence rate of 4.3% to 9.7%. Patients with advanced disease, unmutated immunoglobulin heavy-chain variable region, and high expression of ζ-chain-associated protein kinase 70 (ZAP-70) are also at high risk for developing AIHA [52]. Purine analog-based therapy has been associated with AIHA. Recent studies have also reported a higher incidence of AIHA in patients treated with fludarabine or chlorambucil compared to those receiving R-FC or FC regimens [52,53]. Laboratory results found anemia followed by thrombocytopenia. Clinically autoimmune cytopenia is considered when it appears in sudden hemolytic events, thrombocytopenia of unknown origin without septicemia, or splenomegaly. In most cases, AIHA can be managed with corticosteroids. Intravenous immunoglobulin, cyclosporine, and splenectomy may be used in steroid-refractory cases. If the cause of autoimmune cytopenia is a monotherapy, especially fludarabine monotherapy, the chemotherapy regimen should be changed immediately to a combination regimen [54].
CONCLUSIONS
In Korea, although there are small numbers of patients diagnosed with CLL, the incidence of CLL is gradually increasing. This increase appears to be related with the rapid aging of society and the westernization of Korean lifestyles.
As the rapid evolution of treatment strategies for CLL and development of new drugs warrant a closer look into CLL, Korean medical doctors and researchers should take a profound interest in the field of CLL.
Notes
No potential conflict of interest relevant to this article was reported.