Number of existing permanent teeth is associated with chronic kidney disease in the elderly Korean population
Article information
Abstract
Background/Aims
The aim of this study was to assess the association between the number of existing permanent teeth and chronic kidney disease (CKD) in a representative sample of the elderly Korean population.
Methods
A total of 2,519 subjects who participated in the Korean National Health and Nutrition Examination Survey were cross-sectionally examined. The number of existing permanent teeth was evaluated by clinical oral examination. CKD was defined based on definition and classification by Kidney Disease: Improving Global Outcomes (KDIGO) 2012 guidelines. Multivariable logistic regression analyses were performed controlling for age, gender, income, education, tooth-brushing frequency, periodontitis, state of dentition, smoking, alcohol consumption, hypertension, obesity, diabetes mellitus, and hypercholesterolemia. Subgroup analyses by age and gender were also performed.
Results
The number of teeth was significantly associated with CKD after controlling for all potential confounders (adjusted odds ratio [AOR], 1.67; 95% confidence interval [CI], 1.04 to 2.70 for lower number of teeth; AOR, 1.59; 95% CI, 1.14 to 2.23 for moderate number of teeth). In the subgroup analyses, the association was highlighted in females aged 75 years over (AOR, 2.55; 95% CI, 1.05 to 6.20 for lower number of teeth; AOR, 1.95; 95% CI, 1.01 to 3.80 for moderate number of teeth).
Conclusions
Our findings suggest that the number of existing permanent teeth may be associated with CKD among Korean elderly.
INTRODUCTION
Chronic kidney disease (CKD) is a grave public health issue with adverse outcomes such as hospitalization, kidney failure, cardiovascular diseases and premature mortality [1]. The prevalence of CKD is estimated to be greater than 10% worldwide [1]. According to the updated definition and criteria from the Kidney Disease: Improving Global Outcomes (KDIGO) [2], CKD can be assessed through the estimated glomerular filtration rate (eGFR) and albuminuria stage. In Korea, the prevalence of CKD was reported to be 7.9% for 2011 to 2012, as determined by the Korean National Health and Nutrition Examination Survey (KNHANES) [3]. The higher risk of CKD showed a stronger association in females than males and in the older groups [3].
Periodontitis is a chronic inflammatory disease which leads to irreversible destruction of tissues of the teeth and surrounding structures [4]. It is closely related to several systemic diseases such as atherosclerosis [5] and metabolic syndromes [6]. Complicated outcomes such as severe periodontitis and untreated dental caries are the main causes of tooth loss [7], the prevalence of which greatly increases with age [8]. In addition, the predominance of adults aged 65 years and over with fewer than 19 natural teeth was reported to be approximately 50% in Korea [9].
In a recent systematic review, there was evidence supporting the positive link between periodontitis and CKD and the beneficial effects of periodontal treatment on eGFR [10]. Two cohort [11,12] and 11 cross-sectional studies [13-23] have examined the association between periodontitis and CKD in the United States, Brazil, United Kingdom, Japan, China, and South Korea. Most presented positive results, except for two studies [14,17]. In addition, three reports showed that edentulous adults were significantly associated with CKD [14,20,24].
To date, no previous study has provided information on the association between CKD and the number of existing permanent teeth, which is usually related to oral diseases in the Korean elderly. Thus, we hypothesize that the number of permanent teeth is associated with CKD in the elderly Korean population.
This study aims to assess the association between the number of existing permanent teeth and CKD after considering various confounding variables such as age, gender, income, education, tooth-brushing frequency, periodontitis, state of dentition, smoking, alcohol consumption, hypertension, obesity, diabetes mellitus, and hypercholesterolemia. We also examined the strength of this association within different age and gender subgroups.
METHODS
Study population
The participants were drawn from the KNHANES collected by the Korea Center for Disease Control and Prevention (KCDC) in 2012 to 2014. The sampling protocol for the KNHANES was designed to include a complex, stratified, multistage, and probability-based sampling design with proportional allocation. The participants were randomly selected from diverse geographic areas, age, and gender groups from the 2005 National Census Registry. The survey consisted of questions regarding overall health, nutrition, and health examinations. The national survey was approved by the Institutional Review Board of the KCDC (2012-01EXP-01-2C, 2013-07CON-03-4C, and 2013-12EXP-03-5C). All participants signed an informed consent form before participating in the study. The data for this research were acquired from household interviews, physical and oral examinations, and blood samples obtained from examination centers. Of the 23,626 subjects who participated in the KNHANES, exclusion criteria were as follows: (1) < 65 years of age, (2) missing values in main variables and confounders such as oral examination, laboratory test for kidney function and various health variables. The final sample included 2,519 participants (1,101 males and 1,418 females).
Assessment of chronic kidney disease
The eGFR was calculated from serum creatinine levels using the Modification of Diet in Renal Disease (MDRD) equation: eGFR = 175 × serum creatinine–1.154 × age–0.203 × 0.742 (in females) [25]. The eGFR equation was validated for Asian populations and reported in mL/min/1.73 m2 [26]. eGFR were classified into five groups: eGFR ≥ 90 mL/min/1.73 m2 (G1); eGFR = 60 to 89 mL/min/1.73 m2 (G2); eGFR = 45 to 59 mL/min/1.73 m2 (G3a); eGFR = 30 to 44 mL/min/1.73 m2 (G3b); and eGFR < 30 mL/min/1.73 m2 (G4–5). Urinary albumin to creatinine ratios (UACRs) were calculated in milligrams per gram and divided into three categories by the KDIGO staging system [2]. UACR were classified into three groups: UACR < 30 mg/g (A1); UACR = 30 to 300 mg/g (A2); and UACR > 300 mg/g (A3). CKD was defined using recent criteria that combined groups with eGFR < 60 mL/min/1.73 m2 and UACR ≥ 30 mg/g by the 2012 CKD guideline released by KDIGO [2]. CKD was classified into four categories, those with no risk, moderate risks (G3a–A1 or G1–2 A2), high risks (G3b–A1, G3a–A2, G1–2 A3), and extremely high risks (G4–5 A1, G3b–5 A2, G3a–5A3) [2]. Finally, CKD was dichotomized with a yes or no parameter [27].
Assessing the number of existing permanent teeth
The number of existing permanent teeth was assessed by dentists, which excludes missing teeth, impacted teeth or implants and wisdom teeth. Root tips and teeth indicated for extraction were categorized as missing teeth. The number of existing permanent teeth was divided into three categories: 0 to 9, 10 to 19, and 20 to 28. Han et al. [28,29] recently reported that the cut-off for the number of teeth was 10 and 20 in the Korean population. The results of the oral health status also presented prevalence rates of the elderly who have 20 or more natural teeth through the KNHANES survey [9].
Assessment of potential confounders
Confounding variables used in statistical analyses included information on sociodemographic factors, oral health status and behaviors, and general health status and behaviors. Individual information from participants were gathered from personal interviews using structured questionnaires.
Household income was categorized into quartiles, while educational level was classified into four groups: less than primary school, middle school, high school, and more than college. The daily tooth-brushing frequency was categorized into two groups: less than twice a day and three or more times. Periodontal status and state of dentition were carefully assessed by dentists, in which the Community Periodontal Index of Treatment Needs (CPITN) was used to quantify periodontitis. The ten selected teeth were 11, 16, 17, 26, 27, 31, 36, 37, 46, and 47, according to the World Health Organization guidelines. Community periodontal index (CPI) was scored from 0 to 4: 0 (normal), 1 (gingivitis with bleeding on probing), 2 (presence of calculus), 3 (pocket depth [PD] ≥ 3.5 mm), and 4 (PD ≥ 5.5 mm). Periodontal status was grouped into two categories: absence (CPI 1 to 2) and presence (CPI 3 to 4) of periodontitis, while the state of dentition in the oral cavity was classified into three groups, namely natural dentition, removable partial denture and complete denture. Smoking status was categorized as never/former smokers and current smokers. The frequency of alcohol consumption was categorized into two groups: non-drinkers and drinkers (days per month or week). Hypertension was attributed to people with an average systolic blood pressure over 140 mmHg or diastolic blood pressure over 90 mmHg or medicated for hypertension. Body mass index was calculated as weight divided by the square of the height (kg/m2), and was classified into two groups: normal (< 25 kg/m2) and obese (≥ 25 kg/m2). Diabetes mellitus was defined as having a fasting glucose level over 126 mg/dL or medicated for diabetes. Lastly, hypercholesterolemia was defined as having total cholesterol levels over 240 mg/dL or medicated for hypercholesterolemia.
Statistical analyses
Statistical analyses were performed following the KNHANES guidelines for applying complex survey designs and sampling weights. The outcome variable was CKD and the explanatory variable was the number of teeth. The characteristics of participants with or without CKD were presented using frequency distributions for the categorical variables with a chi-square test. All data were presented as weighted percentages and accompanied with calculations of standard errors. We presented the distribution of kidney function according to the number of teeth using a chi-square test. We then conducted logistic regression analyses to calculate crude odds ratio (OR) and adjusted odds ratio (AOR) with 95% confidence intervals. Multivariable logistic regression analyses were sequentially applied to assess the association between the number of teeth and CKD after adjusting for age, gender, income, education, tooth-brushing frequency, periodontitis, state of dentition, smoking, alcohol consumption, hypertension, obesity, diabetes mellitus, and hypercholesterolemia. Model 1 represents a crude association, while model 2 is adjusted for sociodemographic factors. Model 3 is adjusted for all the variables in model 2, including oral health status and behaviors, and lastly model 4 is adjusted for all the variables in model 3, including general health status and behaviors. We evaluated the fully AOR while adjusting for confounding and other variables. Subgroup analyses by age and gender were also performed to identify risk groups. All analyses were performed using the SPSS version 19.0 (IBM Co., Armonk, NY, USA).
RESULTS
Characteristics of the study participants
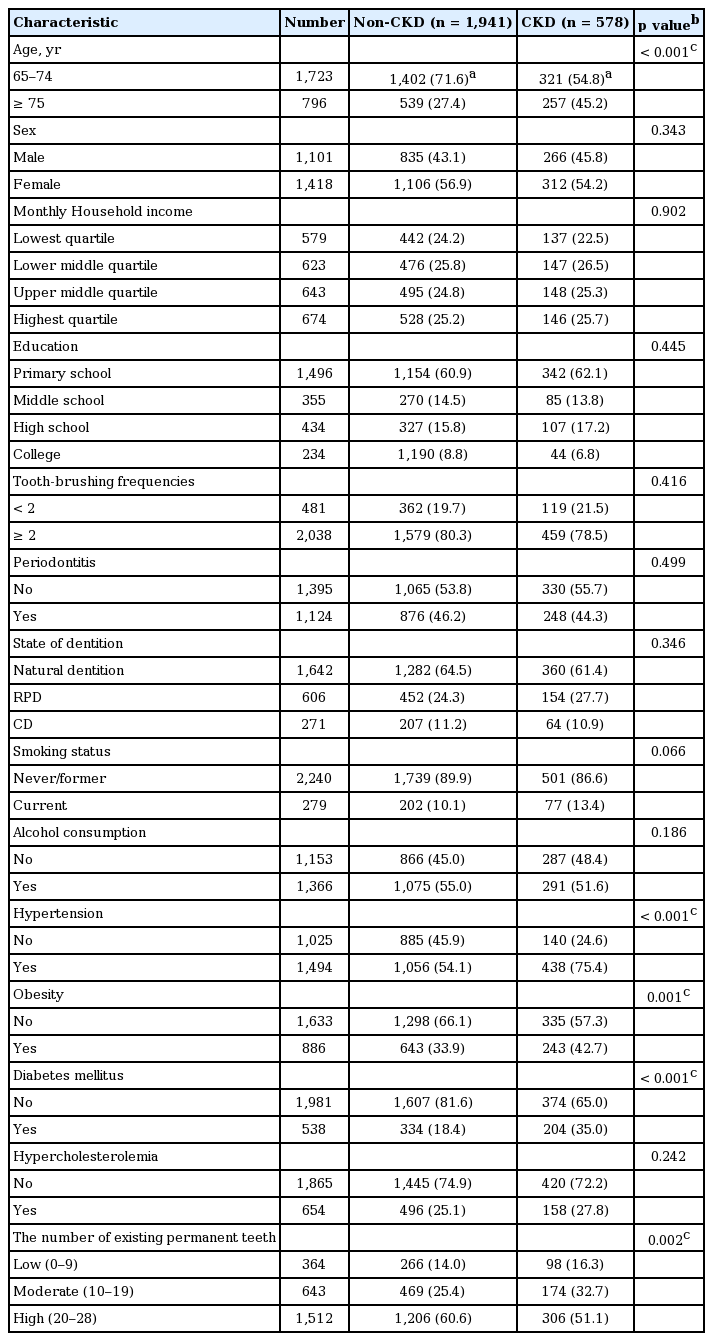
Of the 2,519 participants included in the analyses, 578 (22.9%) were classified with CKD (Table 1). The univariate association between the explanatory and confounding variables and CKD was examined using chi-square tests. The percentage of participants with CKD was significantly higher in the 65- to 74-year-old age group. Hypertension, obesity, and diabetes mellitus were significantly different between CKD and non-CKD groups.
Distribution of CKD as a function of teeth number
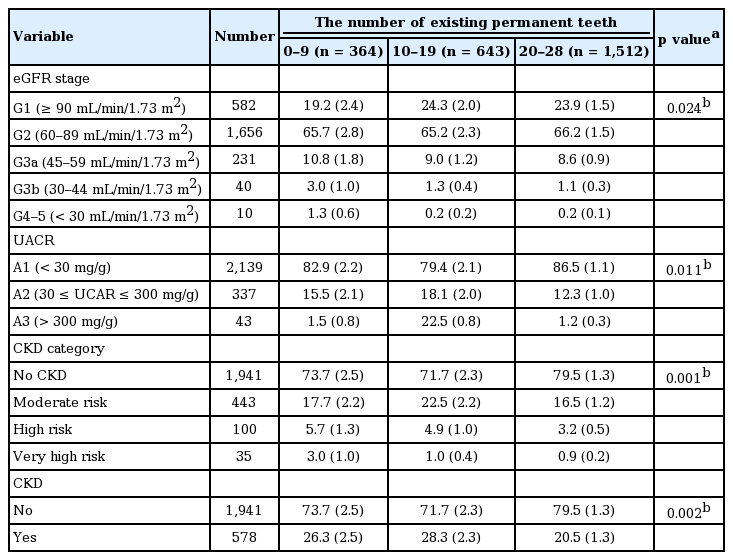
Table 2 shows the distribution of kidney function according to the number of teeth. Participants with fewer teeth had significantly lower eGFR and higher UACR. Consequently, the CKD category was presented with decreased eGFR and increased UACR. Participants with fewer number of teeth were more likely to suffer from a CKD risk when compared to participants with a greater number of teeth.
Association between the number of teeth and CKD
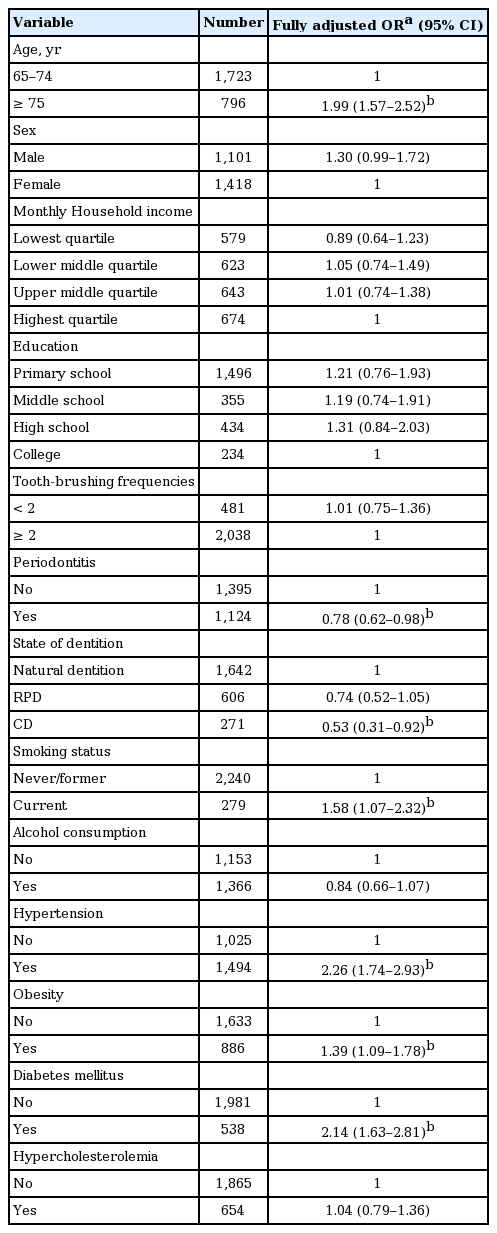
Through a crude analysis, the number of teeth was found to be associated with CKD (Table 3). After adjusting for sociodemographic factors, the association between lower number of teeth and CKD disappeared for model 2. In models 3 and 4, the strength of the association between the number of teeth and CKD remained significant. A dose-effect trend was observed for the association.
Association between CKD and potential confounding variables
We evaluated the AOR of each confounder through a fully adjusted model (Table 4). Confounding variables included participants aged 75 and older, who are currently smoking, hypertension, obesity, and diabetes mellitus associated with CKD. Periodontitis and complete denture were negatively associated with CKD.
Age- and gender-stratified association between the number of teeth and CKD
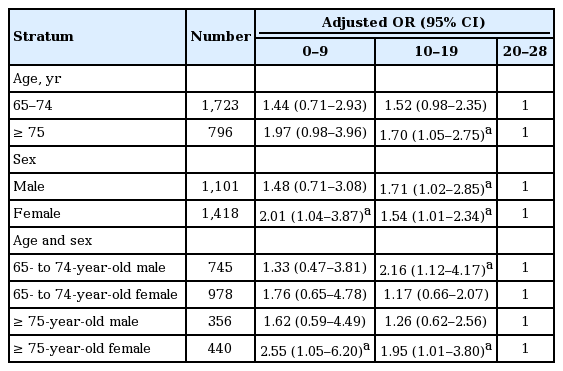
The stratified association between the number of teeth and CKD was highlighted in the specific subgroups (Table 5). In analyses in which age and gender were both taken into consideration, the strength of the link between the number of teeth and CKD specifically increased in females aged 75 years and over.
DISCUSSION
To our knowledge, the findings of this study provide the evidence associating the number of existing permanent teeth and CKD, independent of traditional risk factors such as hypertension, diabetes mellitus, smoking, and various potential confounders.
Although a growing body of evidence have demonstrated an association between periodontitis, edentulism and CKD, the concordance remains controversial [10-23]. Analyses of population-based data from 12,947 participants showed a positive association between edentulism and periodontal disease on CKD in the United States population (OR, 1.60 for periodontitis; OR, 1.85 for edentulism) [15]. Another study presented a positive association between only edentulism and CKD in the National Health and Nutrition Examination Survey (OR, 1.64) [14]. Others have emphasized that individuals with CKD have to recognize the impact of periodontitis on the disease, and the importance of periodontal treatment [30]. Through a United States national survey, periodontitis, and edentulism were identified to potentially increase the mortality of participants with CKD [31]. They suggested that periodontal disease may be a crucial risk factor for cardiovascular diseases and mortality in general for participants with CKD. In contrast, periodontitis is inversely associated with CKD in our study. Although there is only one existing study on the association between periodontitis and CKD in the Korean population [23], the results were contradictory with the present study. The conflicting results may be influenced by age distribution, definition, and criteria of CKD, and confounding variables. Additional studies will be needed to confirm the link between periodontitis and CKD in the Korean population.
The results of this study indicated that lower numbers of teeth had significantly higher AOR than moderate numbers in a fully adjusted model. Our data cannot compare with other studies due to the absence of information linking the number of teeth and CKD. In our subgroup analyses, the link between the number of teeth and CKD showed a stronger association in females aged 75 years and over when compared to the total participants (AOR, 2.55 for lower number of teeth; AOR, 1.95 for moderate number of teeth). We then speculated potential arguments for these results. According to previous findings, age and gender differences exist in the prevalence of tooth loss [9] and CKD [3]. The higher risk for CKD and severe tooth loss showed a stronger association in females than in males, and in the older age group than the younger [3,9]. Therefore, the association between CKD and tooth loss may play a more significant role in females aged 75 years and over than any other group. The causal relationship between the number of teeth and CKD has yet to be established.
The biological reasoning linking tooth loss to decreased kidney function may be explained by a few related theories. Chewing difficulties due to tooth loss may affect food diversity and dietary patterns [32], which can lead to poor nutrition [33]. Nutritional deficiencies may cause weight loss and development of major chronic diseases and cardiovascular diseases [34]. Moreover, dietary patterns are associated with cardiometabolic and endocrine biomarkers. Previous studies demonstrate that the dietary modification were effective in controlling blood pressure and diabetes [35,36]. Chronic diseases such as diabetes mellitus and hypertension are traditional risk factors for the negative consequences associated with CKD. Thus, our study suggests that tooth loss can affect CKD through hypertension and diabetes mellitus, which are important risk factors for CKD.
In this study, the number of existing permanent teeth is an indicator of oral health status, which is primarily examined among elderly populations in oral health research. Kressin et al. [37] reported that tooth-brushing, dental floss use, regular prophylaxis, and combinations of oral health-related behaviors can lead to tooth retention. Our data suggest that managing and maintaining oral health is important throughout life, and that the number of teeth may affect CKD at old age.
That said, there are some limitations to this study. First, since there were no previous studies of the same nature, we could not make a detailed comparison of our findings, and thus additional evidence are needed to clarify the association. Recent studies have reported the impact of tooth loss on dietary intake in individuals with CKD [38], in which nutritional interventions may contribute to the alleviation of CKD occurrence [39]. Thus, further studies should consider the nutritional status of participants as a potential confounding variable. Moreover, because our study was limited to elderly populations, our results cannot be generalized to all age groups. According to previous studies, edentulous participants may have a higher risk for CKD [14,15], as such, it may be necessary to consider edentates separately. Lastly, this cross-sectional study does not have the ability to identify causal relationships; further well-designed longitudinal studies are needed to determine the biological mechanisms for the presented association.
Notwithstanding, our study has major strengths. First, we evaluated the definition and classification system for CKD and applied them through GFR and albuminuria categories as proposed by KDIGO 2012. An updated KDIGO guideline provides relatively easy and accurate methods for early detection of CKD, compared to the CKD-EPI (chronic kidney disease-epidemiology collaboration) equation and MDRD study equation [40]. Second, the number of teeth and oral health status in the entire oral cavity were objectively examined by dentists. Third, general health examination and laboratory analyses were documented by professional examiners. Fourth, the results were acquired from elder participants who represent the Korean population from the KNHANES data. These results provide the first evidence linking the number of existing permanent teeth and CKD among older adults. Thus, medical and dental professionals should be concerned with the association between the number of teeth, as an endpoint of oral health, and CKD.
KEY MESSAGE
1. The number of existing permanent teeth is associated with chronic kidney disease in Korean elderly population.
2. The lower number of existing permanent teeth was high in those with chronic kidney disease. The link was highlighted in females aged 75 years over.
3. Medical and dental professionals should be concerned with the link between the number of teeth and chronic kidney disease at old age.
Notes
No potential conflict of interest relevant to this article was reported.