Utility of shear wave elastography to detect papillary thyroid carcinoma in thyroid nodules: efficacy of the standard deviation elasticity
Article information
Abstract
Background/Aims
The aim of this study was to address the role of the elasticity index as a possible predictive marker for detecting papillary thyroid carcinoma (PTC) and quantitatively assess shear wave elastography (SWE) as a tool for differentiating PTC from benign thyroid nodules.
Methods
One hundred and nineteen patients with thyroid nodules undergoing SWE before ultrasound-guided fine needle aspiration and core needle biopsy were analyzed. The mean (EMean), minimum (EMin), maximum (EMax), and standard deviation (ESD) of SWE elasticity indices were measured.
Results
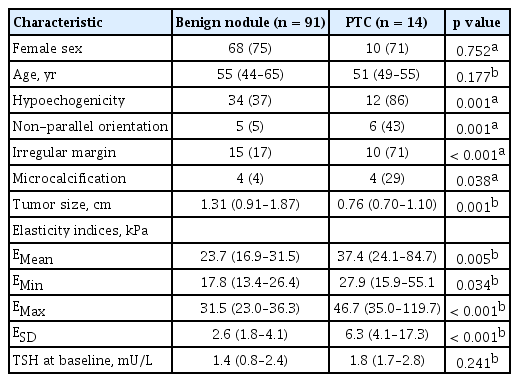
Among 105 nodules, 14 were PTC and 91 were benign. The EMean, EMin, and EMax values were significantly higher in PTCs than benign nodules (EMean 37.4 in PTC vs. 23.7 in benign nodules, p = 0.005; EMin 27.9 vs. 17.8, p = 0.034; EMax 46.7 vs. 31.5, p < 0.001). The EMean, EMin, and EMax were significantly associated with PTC with diagnostic odds ratios varying from 6.74 to 9.91, high specificities (86.4%, 86.4%, and 88.1%, respectively), and positive likelihood ratios (4.21, 3.69, and 4.82, respectively). The ESD values were significantly higher in PTC than in benign nodules (6.3 vs. 2.6, p < 0.001). ESD had the highest specificity (96.6%) when applied with a cut-off value of 6.5 kPa. It had a positive likelihood ratio of 14.75 and a diagnostic odds ratio of 28.50.
Conclusions
The shear elasticity index of ESD, with higher likelihood ratios for PTC, will probably identify nodules that have a high potential for malignancy. It may help to identify and select malignant nodules, while reducing unnecessary fine needle aspiration and core needle biopsies of benign nodules.
INTRODUCTION
The prevalence of thyroid nodules has been rapidly increasing as a result of ultrasound (US) examinations. Thyroid nodules are found in up to 58% to 68% of adults by means of US [1,2]. The overall incidence of malignancy in these nodules has been estimated at only 5% to 10% [2,3] Therefore, it is clinically important to distinguish malignant nodules from benign nodules, which do not require aspiration or surgery.
US elastography is an emerging technique that reflects the stiffness of the lesion. Among the various methods for performing elastography, shear wave elastography (SWE) depends less on the individual operator than strain elastography; thus, more reproducible results can be expected [4]. SWE can be used to evaluate malignancy in many organs such as the liver [5], breast [6], and prostate [7]. Recently, some studies have focused on providing the efficacy and diagnostic accuracy of SWE in the differential diagnosis of benign and malignant thyroid nodules, but have resulted in different SWE parameters with different cut-off values [8-12].
The stiffness within the thyroid nodules depends on the cellularity and composition. Especially, papillary thyroid carcinoma (PTC) has a mixed pathologic structure comprising papillary structures and cystic changes within the mass resulting alternative solid tissue and fluid composition (Fig. 1), which may produce heterogeneous results in elasticity. We hypothesized that focal heterogeneous stiffness of alternating fluid and solid papilla could be quantitatively assessed by using the standard deviation (ESD) of SWE elasticity and help predict PTC. Recent studies have shown that ESD is associated with increased risk of breast cancers [6] and salivary cancers [13]. For thyroid nodules, two previous studies [10,12] tried to examine the utility of ESD and reported conflicting results.
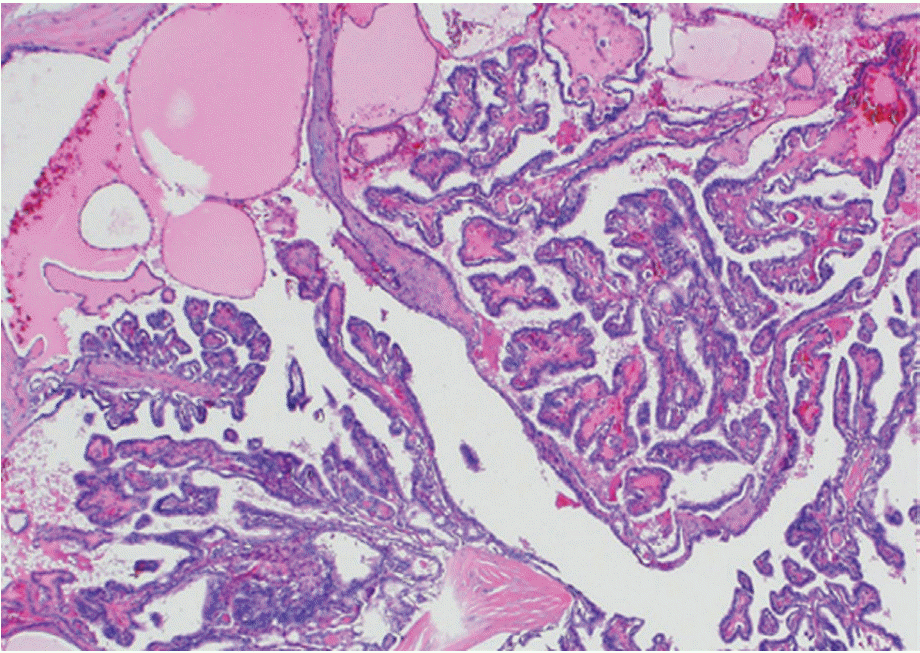
Fingdings on surgical histology. The classical histologicappearance of papillary thyroid cancer with papillarystructure consisting of one or two layers of tumor cellssurrounding a well defined fibrovascular core and cysticchanges (H&E, ×40).
The aim of our study was to address the role of elasticity indices, especially ESD, as a possible predictive marker for PTC and to evaluate the diagnostic performance of SWE for the differentiation of benign and malignant thyroid nodules.
METHODS
Study population
From January 2014 through December 2014, a total of 450 consecutive patients who visited the Soonchunhyang University Seoul Hospital for an evaluation of newly detected thyroid nodules were retrospectively reviewed for this study. Among these subjects, 119 were performed an US-guided fine needle aspiration (FNA) for thyroid nodules. Considering American [14] and Korean [15] management guidelines for patients with thyroid nodules, the FNA criteria for thyroid nodules were as follows: (1) a subcentimeter nodule has one of the findings for malignancy, such as microcalcifications, hypoechogenicity, irregular margins, or a taller-than-wide shape; (2) a patient has a personal history of radiation exposure or familial thyroid cancer; (3) a nodule has indeterminate findings on US > 1 cm in diameter; and (4) a benign appearing nodule, such as a simple cyst or a spongiform nodule, > 2 cm in diameter. For patients who had two or more thyroid nodules, a nodule with suspicious sonographic appearance was aspirated preferentially. If none of the nodules has a suspicious sonographic feature, the largest nodule was aspirated. All patients underwent SWE prior to an US-guided FNA for thyroid nodules. Two experienced pathologists (I.H.C. and S.Y.J.) in the department of pathology confirmed the cytological and/or histological specimens. Fourteen thyroid nodules were excluded because they were characterized, based on the Bethesda system for reporting thyroid cytopathology [16], as non-diagnostic or unsatisfactory (n = 9), or atypia of undetermined significance or follicular lesion of undetermined significance (n = 5). Ultimately, the data from 105 thyroid nodules from 105 patients were included in this study. The diagnosis of PTC was confirmed by histological examination of the surgically resected tumors. The diagnosis of a benign thyroid nodule was confirmed by the histological examination of a surgically resected nodule or by FNA cytology and core needle biopsy results. The requirement for informed consent for this study was waived by the Institutional Review Board because researchers only accessed the database for analysis purposes and because personal identifying information was not accessed. The Institutional Review Board of Soonchunhyang University Seoul Hospital Seoul approved the study (IRB File No. 2016-05-002).
Gray-scale US and SWE examinations
The patients were positioned for US with their necks hyperextended. Each patient underwent gray-scale US and SWE using the Aixplorer US system (SuperSonic Imagine, Aix-en-Provence, France) and a linear probe with a frequency range of 4 to 15 MHz.
During gray-scale US examination, thyroid nodules were evaluated for size (width, depth, and length), volume, composition, orientation, echogenicity, shape, margin, and presence or absence of calcification.
After the gray-scale US, SWE was performed by the same investigator who had performed the gray-scale US and the system was changed to the SWE to use the same probe to perform SWE. Gray-scale US and SWE images appeared simultaneously on two panels (Fig. 2). The built-in region of interest (ROI; Q-box, SuperSonic Imagine) of the system was set to include the nodule and the surrounding normal tissue, which showed the semitransparent color map of the tissue stiffness overlaid on the gray-scale US image. The software generated and automatically calculated the mean (EMean), minimum (EMin), maximum (EMax), and ESD of the lesions’ shear elasticity indices in kPa. A fixed 2 × 2 mm size of ROI was placed by an investigator over the visually stiffer region of the nodule on the color map to obtain the EMax, selecting two or more fixed ROIs depending on the size or extent of the SWE color map. The representative values of the EMean, EMin, EMax, and ESD were obtained from the ROI with the highest EMax. To avoid false results by SWE, macrocalcifications and the isthmic/paraisthmic areas due to the interference produced by the tracheal cartilage were excluded from the ROI [17]. The color map of the tissue stiffness ranged from dark blue (lowest stiffness, 0 kPa) to red (highest stiffness, 180 kPa).
Statistical analysis
The tumor size and elasticity values of all the lesions are expressed as medians (interquartile range). Differences between benign and PTC groups were compared using the Mann-Whitney U test. Group comparisons of categorical variables were performed using the chi-square test. For small cell value, Fisher exact test was used. Results of categorical data are summarized using frequencies and percent values. We evaluated the sensitivity and specificity of the elasticity values to predict PTC using a receiver operating characteristic curve analysis, estimating the area under the curve with 95% confidence intervals. The likelihood ratio demonstrates how many times more (or less) likely the patients with the disease are to have that particular result than patients without the disease [18,19]. Likelihood ratios above 10 and below 0.1 represent strong evidence to rule in or rule out the diagnosis, respectively [18,19]. All statistical analyses were performed using the SPSS version 14.0 (SPSS Inc., Chicago, IL, USA). A p value of less than 0.05 was considered statistically significant.
RESULTS
Comparison of characteristics between patients with benign nodule and PTC
Of the 105 eligible patients, 14 (13%) were diagnosed with PTC by FNA and surgical pathology and 91 (87%) were revealed as benign nodules based on FNA and core needle biopsy results.
We compared the baseline characteristics of the patients with benign thyroid nodules to those with PTCs (Table 1). Gray-scale US features of hypoechogenicity, non-parallel orientation, irregular margin, and microcalcification were significantly more frequently found in PTCs than benign nodules. Median tumor size was larger in benign nodules than PTCs (p = 0.001). PTCs had higher absolute elasticity values than benign thyroid nodules, which were statistically significant for EMean (p = 0.005), EMin (p = 0.034), EMax (p < 0.001), and ESD (p < 0.001) (Fig. 3). There were no significant differences between the groups with respect to gender, age at diagnosis, or serum thyroid stimulating hormone levels.
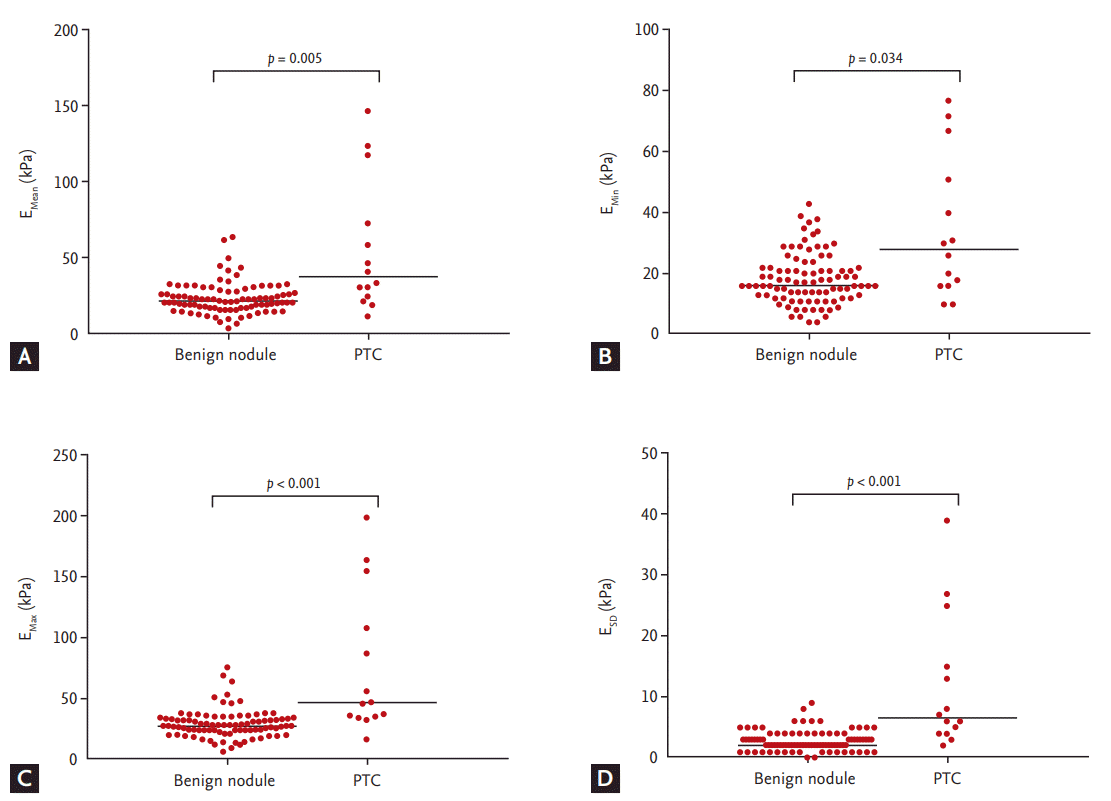
Scatter dot plots of shear wave elastography measurements for benign nodules (n = 91) and papillary thyroid carcinomas(PTC, n = 14). (A) Mean elasticity (EMean). (B) Minimum elasticity (EMin). (C) Maximum elasticity (EMax). (D) One standarddeviation of elastographic values (ESD). Cental bars denote median.
Diagnostic performance of elasticity indices for PTC
The receiver operating characteristic analysis and diagnostic performance of elasticity indices for discrimination between benign nodules and PTCs are presented in Table 2. All elasticity indices were significantly associated with PTC and had diagnostic odds ratios ranging from 3.69 to 14.75. The sensitivity of elasticity indices associated with PTC was somewhat low, ranging from 50.0% to 57.1%. However, the specificity of elasticity indices associated with PTC was high, ranging from 86.4 to 96.6%. The positive predictive value ranged from 46.7% to 77.8%, and the negative predictive value from 87.9% to 89.5%. The positive likelihood ratio ranged from 3.69 to 14.75, and the negative likelihood ratio from 0.49 to 0.57. The ESD showed the best diagnostic accuracy when applied at a cut-off value of 6.5 kPa, which had a specificity of 96.6%, positive likelihood ratio of 14.75 and diagnostic odds ratio of 28.50.
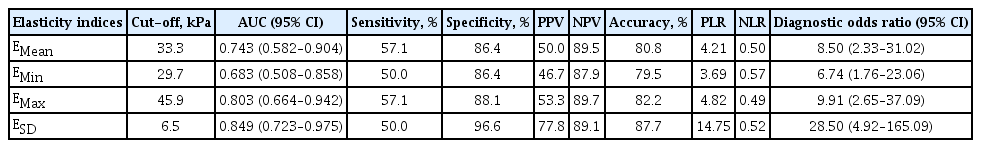
Receiver operating characteristic results and diagnostic performance of elasticity indices for predicting papillary thyroid carcinoma
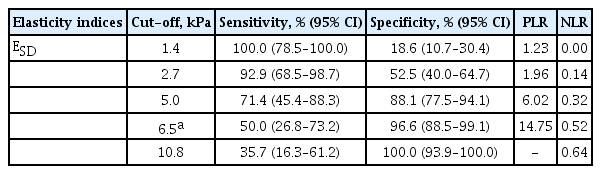
We further analyzed the diagnostic efficacy of the elasticity indices according to ESD values (Table 3). A cutoff of ESD > 1.4 kPa achieved 100% sensitivity but only 18.6% specificity. Conversely, a cut-off of ESD > 10.8 kPa achieved 100% specificity but only 35.7% sensitivity.
DISCUSSION
In the present study, we confirmed that the EMean and EMax of SWE elasticity indices were significantly higher for PTCs than for benign thyroid nodules. This is similar to observations reported by several previous studies. Considering the pathologic structure of PTCs, which usually consists of papillary structures of tumor cells and cystic changes within the tumor, we expected that SWE would alternatively measure solid papilla and cystic fluid over short distances (less than 1 mm). We found that the shear elasticity index of ESD of PTCs were significantly higher than that of benign thyroid nodules. Moreover, the ESD analyzed alone had a clinically relevant positive likelihood ratio of 14.75 to suggest malignancy.
Several US features, such as microcalcifications, hypoechogenicity, irregular margins, and a taller-thanwide shape, have been associated with malignancy [20]. Diagnostic sensitivity ranges from 26.1% to 59.1% for microcalcifications, 26.5% to 87.1% for hypoechogenicity, 17.4% to 77.5% for irregular margins, and 32.7% for the taller-than-wide shape, whereas specificity ranges from 85.5% to 95.0%, 43.4% to 94.3%, 38.9% to 85.0%, and 92.5%, respectively [20]. The positive likelihood ratio was 3.26, 2.99, and 8.07 [19], respectively. More recently, several studies evaluating the diagnostic values of SWE have suggested that the shear elasticity index of EMean may be a useful tool for discriminating between PTCs and benign nodules. However, the cut-off values for malignancy were slightly different among the various studies. Park et al. [11] reported that EMean levels greater than 85.2 kPa provided a sensitivity of 95.0% and a specificity of 56.7% and had a 3.071-fold higher risk of PTC. A pilot study by Sebag et al. [8] demonstrated high diagnostic efficacy for an EMean cut-off value of 65 kPa, with a sensitivity of 85.2% and a specificity of 93.9%. Veyrieres et al. [9] also found, with excellent reproducibility, that the 66 kPa threshold in SWE was the best ultrasound sign to rule out malignant thyroid nodules with a sensitivity of 80% and a specificity of 90.5%. On the other hand, Bhatia et al. [10] showed that EMean levels greater than 34.5 kPa attained a sensitivity of 76.9% and a specificity of 71.1% with cut-off values that were lower than those reported by other studies [8,9,11]. In our current study, the cut-off value of EMean with an optimal sensitivity of 57.1% and specificity of 86.4%, was 33.3 kPa; this is similar to the cut-off values reported by Bhatia et al. [10] The reason for the discrepancy in performance of SWE between the previous studies [8,9,11] may be due to variable inclusion of the study samples with different types of thyroid cancer. In addition, the present finding of lower cut-off values may be partly influenced by potential confounding factors of SWE such as tumor size [10], macrocalcifications [17], cystic changes, the influence of precompression [21], or position.
Heterogeneous stiffness could be quantitatively evaluated by SWE by estimating Young’s modulus in kPa. We hypothesized ESD would improve the accuracy of assessing the PTC, which demonstrates a mixed pathologic structure comprised of papillary structures of tumor cells and cystic changes within the tumor. In accordance with our expectations, the ESD values showed diagnostic accuracy and reproducibility better than EMean and a clinically relevant positive likelihood ratio of 14.75 for malignancy. Heterogeneity has been used to aid benign/malignant differentiation using grayscale US for many years [22]. Recent studies have shown that ESD is associated with increased risk of breast cancers [6] and salivary cancers [13]. For thyroid nodules, two previous studies examining the ESD reported conflicting results. Bhatia et al. [10] did not find that ESD was sufficiently predictive of malignancy. Conversely, Wang et al. [12] showed that US yielded the highest sensitivity and lowest specificity for malignancy when gray-scale US combined with the ESD cut-off value of 6.8 kPa for the nodule. In this study, the ESD of 6.5 kPa was the most accurate threshold, giving a relatively low sensitivity of 50.0% but a high specificity of 96.6%, a positive likelihood ratio of 14.75, and a diagnostic odds ratio of 28.50. The ESD cut-off value of 6.5 kPa could provide strong evidence for confirmation of a PTC diagnosis (likelihood ratio above 10). The inconsistent results are likely due to an analysis in a heterogeneous group of patients with thyroid cancers, including follicular, anaplastic, and medullary cancer, and/or lymphoma, in the previous studies [10,12].
The main limitation of our current study is its retrospective nature. There might have been selection bias because we only included thyroid nodules that underwent US-FNA. This is a single-center study with a limited number of patients. With the lack of multicenter evaluation, large prospective studies evaluating SWE parameters are anticipated to help verify our results, as well as, the impact of SWE on the need for a thyroid FNA.
In conclusion, ESD, a shear elasticity index, had the highest diagnostic performance, among all the SWE parameters, for detection of PTC in thyroid nodules. The ESD could be helpful for identify nodules with an increased risk for PTC, as well as help select which nodules require FNA in addition to the conventional US findings.
KEY MESSAGE
1. Among all the shear wave elastography parameters, standard deviation (ESD) had the highest diagnostic performance for detection of papillary thyroid carcinoma (PTC) in thyroid nodules.
2. The ESD could be helpful for identify nodules with an increased risk for PTC, as well as help select which nodules require fine needle aspiration in addition to the conventional ultrasound findings.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was supported by the Soonchunhyang University research fund.