Ezetimibe decreased nonalcoholic fatty liver disease activity score but not hepatic steatosis
Article information
Abstract
Background/Aims
A number of clinical trials reported varying effects of cholesterol lowering agents in nonalcoholic fatty liver disease (NAFLD) patients. We, therefore, assessed the changes in hepatic steatosis and NAFLD activity score (NAS) after treatment with cholesterol lowering agents in NAFLD patients by metaanalysis.
Methods
The Cochrane Library, the MEDLINE, and the Embase databases were searched until May 2015, without any language restrictions, for randomized controlled trials (RCTs) and nonrandomized studies (NRSs). Additional references were obtained from review of bibliography of relevant articles. The quality of evidence was assessed using the grading of recommendations assessment, development and evaluation guidelines.
Results
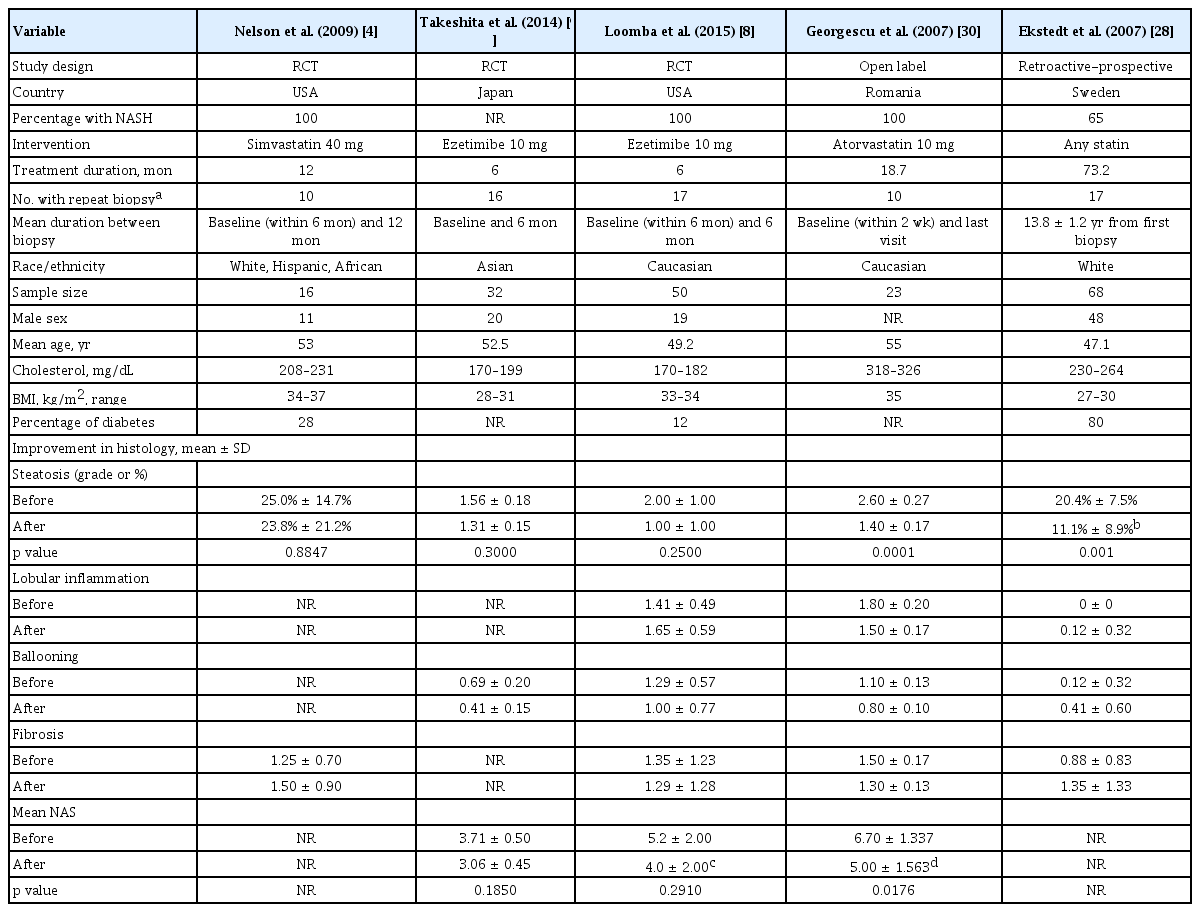
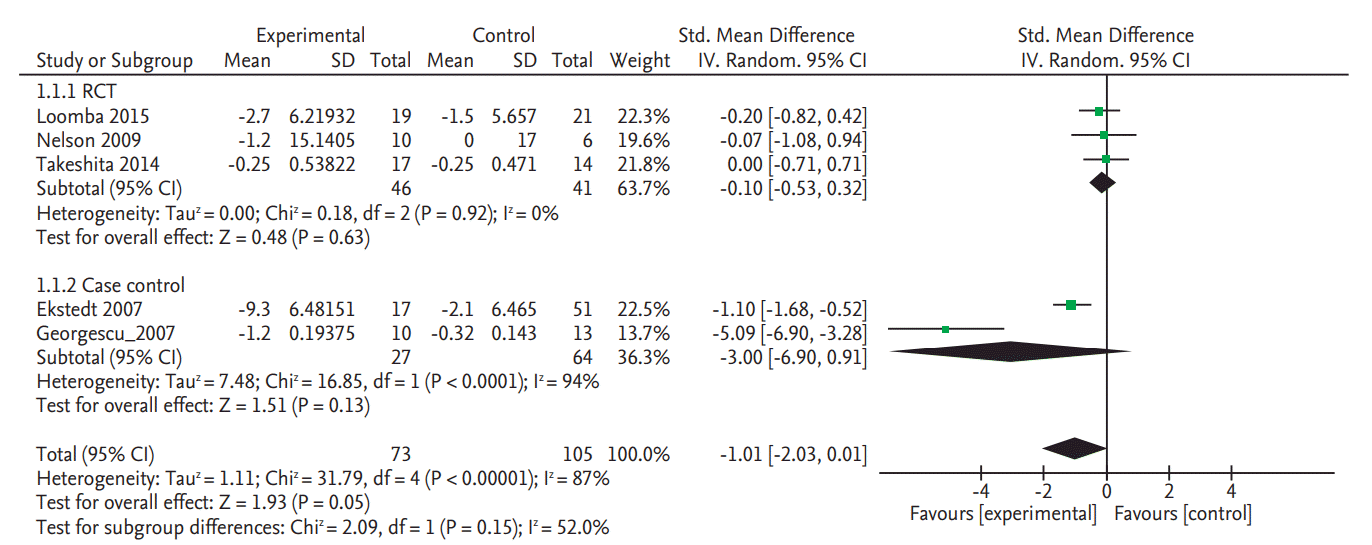
Three RCTs (n = 98) and two NRSs (n = 101) met our study inclusion criteria (adult, NAFLD, liver biopsy). Liver biopsy was performed in all five studies, but only the three studies reported NAS. Ezetimibe significantly decreased NAS (standardized mean difference [SMD], –0.30; 95% confidence interval [CI], –0.57 to –0.03) but not hepatic steatosis in RCT (SMD, –0.1; 95% CI, –0.53 to 0.32), while the effect was significant for both NAS and intrahepatic content in NRSs (SMD, –3.0; 95% CI, –6.9 to 0.91).
Conclusions
Ezetimibe decreased NAS without improving hepatic steatosis.
INTRODUCTION
Metabolic syndrome is a major risk factor for nonalcoholic fatty liver disease (NAFLD), with approximately half of all NAFLD patients also having hypercholesterolemia [1]. Current treatment for NAFLD consists largely of lifestyle modifications and treatment of comorbid conditions such as hyperlipidemia. Experimental studies in mice have shown that ezetimibe and statins not only reduce hepatic inflammation but also fibrosis [2]. Several studies also suggested that hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors may improve liver function tests and histology of NAFLD patients [3,4].
Results from both randomized control trials (RCTs) and non-RCT studies (NRSs) on the effects of cholesterol lowering agents have been difficult to interpret due to the variations in study designs, diagnostic criteria and types of cholesterol lowering agents used. For instance, a sub-study of the St. Francis heart study of 455 subjects showed that, 20 mg of atorvastatin combination with vitamins effectively reduced the odds of developing hepatic steatosis by 71% in healthy individuals as well as those with NAFLD [5]. Another study by Park et al. [6] which included 45 subjects showed ezetimibe as a promising agent for the treatment of NAFLD; however, this study did not have a control arm. In addition, most existing investigations were case control studies [7], and there are currently only four RCTs examining this important issue [4,8,9].
A recent Cochrane systematic review in 2013 identified only two RCTs with a total 205 participants, and neither study evaluated the histological response to statin therapy [7]. The authors concluded that there were insufficient evidence to either support or refute the use of statins in patients with NAFLD.
In the present study, we investigated the efficacy of cholesterol lowering agents in biopsy-proven NAFLD patients. Primary outcome was changes in hepatic steatosis, while the secondary outcomes were improvements in NAFLD activity score (NAS) as assessed by liver biopsy.
METHODS
Data source and literature source
Two investigators independently searched MEDLINE (January 1, 1946 to May 30, 2015), Embase (January 1, 1947 to May 30, 2015) and the Cochrane Central Register of Controlled Trials (CENTRAL; January 1, 1966 to May 30, 2015) without language or publication year restriction.
The following keywords, MeSH and free text were searched through MEDLINE: NAFLD, statin, and ezetimibe (Supplementary Table 1). Bibliographies of potentially relevant articles were manually reviewed to identify additional relevant studies. The identified articles were assessed individually for inclusion (Supplementary Table 2).
Study selection
The studies were initially abstracted if they included the following keywords: NAFLD, statin, cholesterol lowering agent, ezetimibe. For inclusion, the studies were independently selected by two stages of screening using the Population Intervention Comparison Outcome framework [10]. Since the study objective was the histological effect with the lipid lowering agents, only those studies with liver biopsy results for diagnosis of NAFLD and post-treatment were included [11,12]. The required intervention included HMG-CoA reductase inhibitors or ezetimibe which can be administered at any dose for at least 6 months. The control group received no lipid lowering intervention or placebo, and there were no change of weight in all studies. The primary endpoint was improvement in hepatic steatosis while the secondary endpoint was improvement of NAS and safety.
Data extraction
Using a pre-defined data extraction form, two reviewers (H.Y.L. and D.W.J.) independently extracted data from each study. Any disagreement was independently reviewed by a third reviewer (H.J.K.). The following variables were extracted from the selected studies: (1) hepatic steatosis as evaluated by liver biopsy and/or quantitative fat measurement by magnetic resonance imaging (MRI); (2) NAS measurement before and after therapeutic intervention. All outcomes were assessed by differences between treated and control groups. The results were expressed as mean and standard deviations.
Assessment of study methodological quality
Two reviewers (H.Y.J. and D.W.J.) independently assessed the methodological qualities of included studies. The study quality was evaluated using the risk of bias by Cochrane for RCTs (Supplementary Fig. 1) and Newcastle Ottawa scale for NRSs (Supplementary Table 2) [13]. Any unresolved disagreements between reviewers were resolved by the third author (H.J.K.). Publication bias was not assessable due to the small numbers of studies.
Statistical analysis
We analyzed continuous data using standardized mean difference to combine trials that measure the same outcome but utilized different methods. The primary outcome was change of hepatic steatosis by liver biopsy (and MRI quantification in one study). The histological grading in NAFLD, inflammation, and fibrosis was based on scoring systems by either Brunt et al. [14] or Kleiner [15]. Secondary outcome were changes in NAS.
To assess for heterogeneity, we estimated the proportion of between-study inconsistency due to true differences between studies (rather than differences due to random error or chance) using the I2 statistic, with values of 25%, 50%, and 75% considered low, moderate, and high, respectively. Outcomes were analyzed using random effects model and standardized mean difference (SMD) to assess changes in measurements made by different scales. All analyses were performed using RevMan version 5.2 (http://community.cochrane.org/). This meta-analysis was conducted according to the Preferred Reporting Items for Systematic Revise and Meta-Analyses (PRISMA) statement.
RESULTS
Identification of studies
Fig. 1 shows the details of literature research and selection process of the meta-analysis. The initial search strategy identified 857 articles. Of these, 667 publications were excluded after reviewing the title and abstract which indicated that they did not fulfill the selection criteria (Supplementary Table 3). For the remaining 39 articles [4,6-11,13-44], we performed full manuscript review and identified five relevant studies (three RCTs and two NRSs) to include in this meta-analysis.
Study characteristics and patient populations
Table 1 describes characteristics of the five included studies. The five studies comprised a total of 199 participants who received either statins (n = 47) or ezetimibe (n = 42) in the treatment group, and placebo (n = 97) or ursodeoxycholic acid (UDCA) (n = 13) in the control group. Ezetimibe was administered for 6 months and statins was administered from 6 months to 6 years [8,9]. Two studies were conducted in the USA, and one each in Japan, Sweden, and Romania. The study by Nelson et al. [4], Ekstedt et al. [28] and Georgescu and Georgescu [30] used statins, while the study by Loomba et al. [8] and Takeshita et al. [9] used ezetimibe as the lipid lowering agent. By inclusion criteria, liver biopsy was performed in all five studies, but magnetic resonance spectroscopy was also used in one study [8]. The control subjects received placebo except for those in the study by Georgescu and Georgescu [30] which used UDCA. In terms of race/ethnicity, three studies [4,28,30] included mostly or all Caucasian, while two studies [8,9] included mostly or all Asians. In both studies that used ezetimibe for 6 months, the baseline cholesterol level was within normal range (180 to 190 mg/dL). However, in the study by Georgescu and Georgescu [30] in which statin was used, the baseline cholesterol levels were high with mean level ranging 318 to 326 mg/dL. Loomba et al. [8] studied Caucasian subjects (baseline BMI, 33 to 34 kg/m2), while Takeshita et al. [9] studied East Asian subjects with lower BMI (baseline BMI, 28 to 31 kg/m2).
Quality of the studies
Among the three RCTs, the quality of two studies [8,9] was satisfactory, but one study [4] did not have random allocation sequence, optimal allocation concealment, and detailed data description. However, treated and control groups in all three RCTs were well-matched based on baseline characteristics with well-defined treatment response. In the two NRSs, subjects in the two groups were not well-matched since they were not randomized. The level of evidence and grade of recommendation for each outcome are summarized in Supplementary Table 2.
Hepatic steatosis
Cholesterol lowering agents did not significantly decreased the hepatic steatosis in NAFLD patients in the three RCTs (SMD, –0.10; 95% confidence interval [CI], –0.53 to 0.32) or in two NRSs (SMD, –3.00; 95% CI, –6.90 to 0.91) (Fig. 2).
Nonalcoholic fatty liver diseases activity score
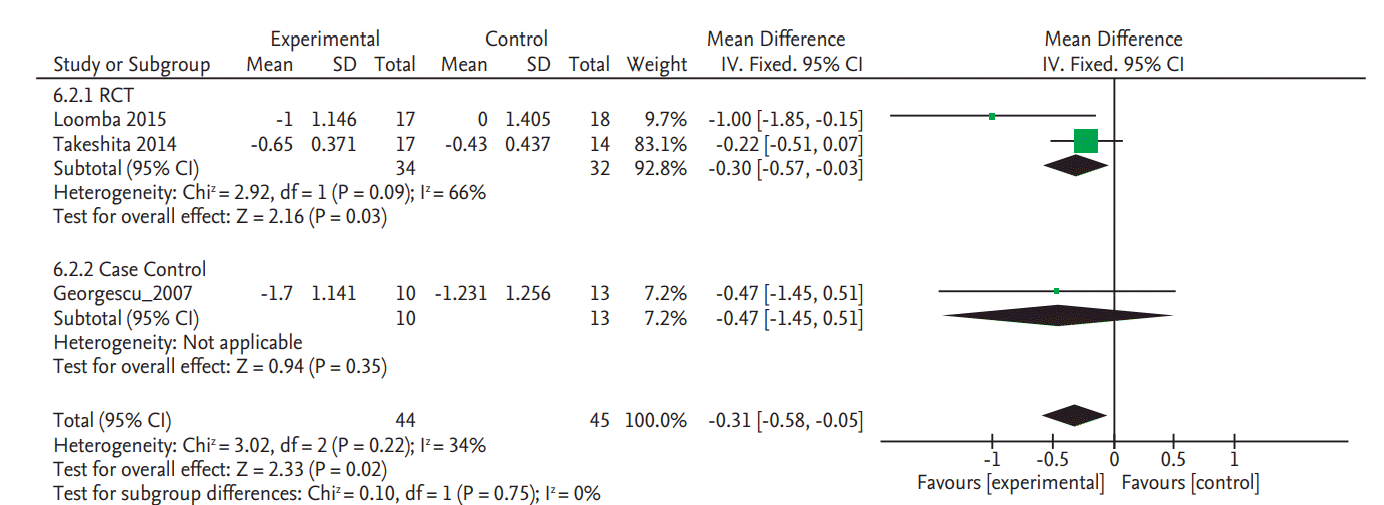
Only two RCTs and one NRS reported the NAS data. Meta-analyzed result of the two RCT studies demonstrated a significant improvement of NAS (SMD, –0.30; 95% CI, –0.57 to –0.03). As shown in Fig. 3, pooled estimate of all three studies with available data also showed significant improvement. However, the mean reduction of NAS was modest: –1.0 in in Loomba et al. [8] and –0.65 in Takeshita et al. [9] but slightly higher in the case control study by Georgescu and Georgescu (mean, –1.7) [30].
Safety
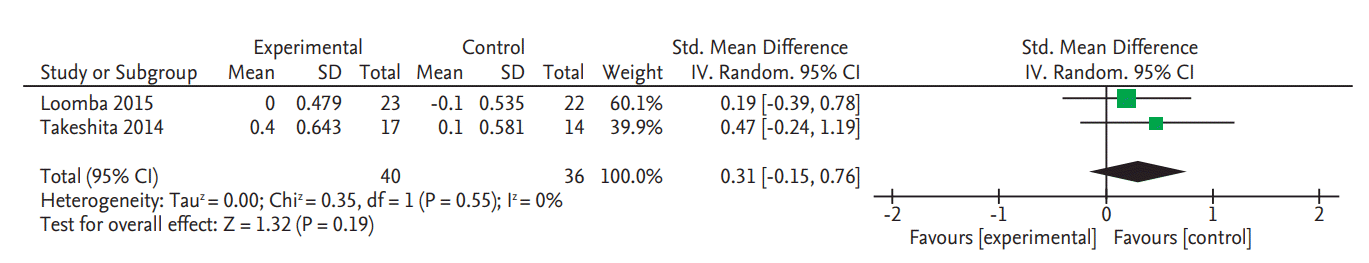
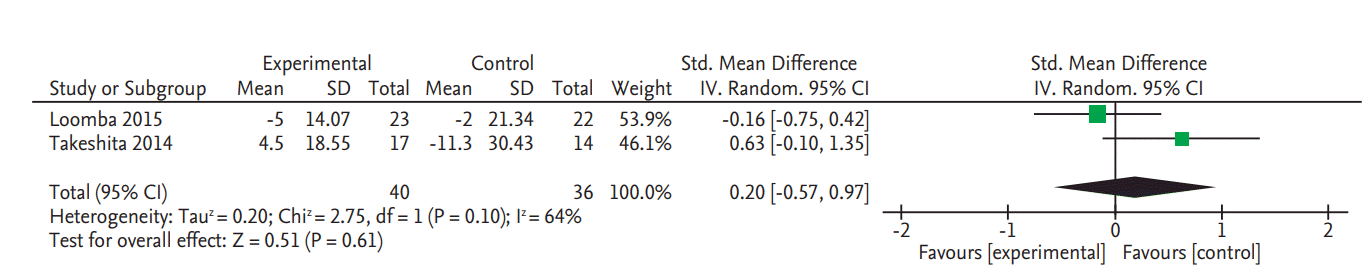
There was no significant change in serum fasting glucose levels in the two RCTs (SMD, 0.20; 95% CI, –0.57 to 0.97) (Fig. 4) [8,9]. Data on glycated hemoglobin (HbA1c) changes were also only reported in these two RCTs and there was no significant change (SMD, 0.31; 95% CI, –0.15 to 0.76) (Fig. 5) [8,9].
Forest plot for decrease of serum fasting glucose. Std., standardized; SD, standard deviation; IV, interval variable; CI, confidence interval.
DISCUSSION
Our meta-analysis showed that ezetimibe decreased NAS (SMD, –0.30; 95% CI, –0.57 to –0.03) without observable improvement in hepatic steatosis. A recent systematic review suggested that statin therapy may improve serum aminotransferase and ultrasound findings [7]. The fundamental differences between our meta-analysis and the previous systemic review were the quantitative methods used for assessment of hepatic steatosis. Our meta-analysis is based only on biopsy, and in one study also MRI-estimated proton density fat fraction (MRI-PDFF) to quantify hepatic fat contents. Contrary to the previous systemic review which included sonography studies for hepatic steatosis assessment, we excluded studies using the sonographic method because it is subjective and poorly quantifiable [11]. Computed tomography (CT) scan is also a less sensitive method to diagnose fatty liver [12], and is therefore a subjective method to estimate quantitative changes in intrahepatic fat content. Thus, we excluded sonographic and CT-based studies in our meta-analysis [32]. Recent data showed that MRI-PDFF has become the primary imaging modality to assess intrahepatic fat content due to their high correlation with liver histology [45], and its clinical use has also been approved by the Food and Drug Administration (FDA) in the USA MRI-PDFF has emerged as a reference standard to measure hepatic steatosis in the radiation zone and is used as the primary modality for endpoint measurement in several clinical trials [45].
Of the RCTs only the two studies which used ezetimibe included NAS data. These two studies showed an improvement in NAS. The study by Loomba et al. [8] used both MRI-PDFF and liver biopsy. In fact, the primary end point of the study was changes in hepatic steatosis as measured by MRI-PDFF, with paired liver biopsy performed in 77.8% of study subjects [8]. We analyzed this study as their biopsy results were well-matched with similar baseline NAS in both groups (5 points each) as well as similar proportion lost at follow-up in both groups (32% vs. 28%, respectively). Moreover, baseline characteristics were also similar in both groups with regards to cholesterol levels, sex and age distribution. These two studies showed a trend of improvement in alanine aminotransferase (ALT) but there was no significant improvement when all four studies with information on ALT were included in the meta-analysis (Supplementary Fig. 2).
Several recent studies have suggested that long-term use of statins could increase the risk of diabetes mellitus and raise serum glucose levels [46]. In this meta-analysis, we did not find any significant increase in fasting serum glucose or HbA1c levels following statin use for 6 months. Takeshita et al. [9] reported a significant increase in HbA1c following the treatment; however, there was no significant increase in HbA1c observed when improvement rate was analyzed. Moreover, statin administration was also not associated with liver toxicity (Supplementary Fig. 2).
This meta-analysis had several limitations. First, the number of included studies was small. As such, we had to pool two cholesterol lowering agents (statin and ezetimibe) together in our analysis. Even though both have lipid-lowering effects, their mechanism of action is different. Second, there is considerable heterogeneity in design and endpoints among the available studies. For example, only three of the five studies were RCTs. Third, there were considerable heterogeneity in the two NRSs. The study by Ekstedt et al. [28] found a greater improvement in hepatic steatosis but the mean baseline cholesterol level was higher in the ezetimibe intervention group than the control at, 264 mg/dL vs. 230 mg/dL (p = 0.04), respectively. In the study by Georgescu and Georgescu [30] the improvement of hepatic steatosis was also observed in the control group who were however treated with UDCA administration.
In summary, the current meta-analysis found that lipid lowering agents can improve NAS in subjects with NAFLD but effects in hepatic steatosis were not observed. Given the small number of available RCTs, as well as a small number of study subjects in interventional studies overall, further large scale RCTs are needed to effectively evaluate the effects of cholesterol-lowering agents in improving intrahepatic fat in NAFLD patients with high baseline cholesterol levels.
KEY MESSAGE
1. Ezetimibe decreased nonalcoholic fatty liver disease (NAFLD) activity score without improving hepatic steatosis.
2. Cholesterol lowering agents did not significantly decreased the hepatic steatosis in NAFLD patients.
3. There was no significant increase in glycated hemoglobin levels following statin use for 6 months.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This work was supported by a research fund from Hanyang University (HY-2014-C).