The efficacy and safety of irsogladine maleate in nonsteroidal anti-inflammatory drug or aspirin-induced peptic ulcer and gastritis
Article information
Abstract
Background/Aims
Irsogladine maleate, an enhancer of gastric mucosal protective factors, has demonstrated its efficacy for various gastric mucosal injuries. The aim of this study was to evaluate the efficacy and safety of irsogladine for prevention of nonsteroidal anti-inflammatory drugs (NSAIDs) or aspirin-induced peptic ulcer and gastritis.
Methods
In this multicenter, randomized, double-blind, exploratory clinical trial, 100 patients over 50 years of age who needed continuous NSAIDs or aspirin for more than 8 weeks were randomly assigned to either test group (irsogladine maleate 2 mg, twice daily, 39 patients for full analysis) or placebo group (37 patients for full analysis). Primary outcomes were incidence of peptic ulcer and ratio of modified Lanza score (MLS) 2 to 4. Secondary outcome was the number of acute erosions confirmed by endoscopy at 8 weeks. Adverse effects were also compared.
Results
There were no significant differences in gastric protective effects between test and placebo groups. However, two cases of peptic ulcer in the placebo group but none in the test group were observed. These two cases of peptic ulcer were Helicobacter pylori-negative. In addition, H. pylori-negative group showed significant changes in MLS score (p = 0.0247) and edema score (p = 0.0154) after the treatment compared to those before treatment in the test group. There was no significant difference in adverse events between the two groups.
Conclusions
The efficacy of irsogladine maleate was found in H. pylori-negative group, suggesting its potential as a protective agent against NSAIDs or aspirin-induced peptic ulcer and gastritis.
INTRODUCTION
Nonsteroidal anti-inflammatory drugs (NSAIDs) are widely used for treating various types of acute and chronic musculoskeletal disorders. According to the Korean National Statistical Office’s data on population distribution by age group, the elderly people aged 65 and over accounted for 13.8% of the total population in 2017. It is expected to increase to 24.3% in 2030 and 37.4% in 2050. When the population is getting older, the number of patients with chronic diseases such as musculoskeletal diseases and cerebrovascular diseases will increase. Therefore, the use of NSAIDs or aspirin is expected to continue to increase. The widespread use of NSAIDs raises the risk of drug side effects, including gastrointestinal (GI) damage. The risk of gastric mucosal defects (erosion or petechiae) in patients with long-term use of NSAIDs has been reported to be about 50%. The rate of ulcers is about 1% and the rate of ulcers with complications is reported to be up to 0.5% per year [1,2]. However, in patients who have previous history of peptic ulcers with old age of more than 65 years old and concurrent use with other NSAIDs or high dose NSAIDs, steroids, and other anticoagulants [3], peptic ulcer can occur frequently [4]. High frequency of peptic ulcer and its complications in aged people [5,6] is caused by decreased gastric mucosal protection provided by connective tissue due to accumulation of oxidative products when people are getting old [7]. In addition, impairment of apoptosis, angiogenesis, and sensory neuron activity via activation of early growth response-1 (Egr-1), phosphatase and tension homologue deleted on chromosome 10 (PTEN) might increase the susceptibility of gastric mucosa to injury during aging [7]. Thus, misoprostol and proton pump inhibitor (PPI) preparations are usually recommended to prevent NSAID or aspirin-induced peptic ulcer and gastritis [8], especially for the elderly. Infection by Helicobacter pylori is more likely to cause peptic ulcers if patients take NSAIDs. Therefore, those who take longterm NSAIDs are recommended to treat H. pylori eradication [9]. However, H. pylori eradication alone cannot reduce the incidence of ulcers [10]. In addition, aspirin is widely used in diseases such as cardiovascular thrombosis and rheumatoid arthritis. When taken daily at low doses, aspirin may damage the mucous membrane of the stomach and cause ulcers or bleeding [11-15]. Treatment guidelines of NSAIDs-induced GI injury are based on prevention of peptic ulcer and gastritis. The most effective method of primary prevention for peptic ulcer caused by NSAIDs is to stop using the drug or not using the drug. However, most patients are unable to discontinue the medication. Therefore, it is important to find strategic preventive measures through risk assessment [16]. In order to prevent the development of peptic ulcer caused by NSAIDs or aspirin, histamine 2 receptor antagonist (H2RA) alone is less effective compared to simultaneous administration of misoprostol or PPI. Misoprostol is effective for preventing GI complications caused by NSAIDs. However, it can cause GI complications such as diarrhea accompanied by convulsive abdominal pain. In addition, it has recently been reported that long term use of PPI can cause various side effects [17], and there are limitations in terms of insurance coverage for long-term prophylactic use. Therefore, developing gastroprotective drugs useful for preventing GI complications due to long-term use of NSAIDs or aspirin is essential.
Irsogladine maleate (Gaslon N® OD, Taejoon Pharm Co. Ltd., Seoul, Korea) is an enhancer of gastric mucosal protective factors. It increases the production of intracellular cyclic adenosine monophosphate (cAMP) by inhibiting phosphodiesterase activity [18]. It can activate intercellular communication [19], prevent reduction in gastric mucosal blood flow [20,21], increase anti-inflammatory activity [18], and prevent reduction in mucosal hydrophobicity [22]. Although its usefulness has been demonstrated in various models of gastric mucosal injury, its efficacy of protection for NSAIDs or aspirin-induced GI damage, especially in people with old age, is currently unknown. Therefore, the objective of this study was to evaluate the efficacy and safety of irsogladine maleate against peptic ulcer and gastritis caused by long-term use of NSAIDs or aspirin in people who were over 50 years old.
METHODS
Study participants
Eligible patients were more than 50 years old who were taking NSAIDs (including cyclooxygenase 2 [COX-2] inhibitor, multiple NSAIDs) or aspirin (more than 80 mg/day) for more than 8 weeks. They had baseline endoscopic findings without active peptic ulcer or gastric erosions. Written informed consent was obtained from each patient before participating in this study.
Exclusion criteria were history of gastric acid inhibiting or esophagogastric surgery, Zollinger-Ellison syndrome, active peptic ulcer or active erosive gastritis at screening endoscopy, reflux esophagitis more than LA-A, bleeding tendency or coagulopathy, inflammatory bowel disease, malabsorption and esophageal stricture within 3 months, hypersensitivity to irsogladine maleate, any use of PPI, H2RA, muscarinic receptor antagonist, corticosteroid, antacid, mucoprotective agents, anticoagulants (except aspirin), anti-thrombotic agents, bisphosphonate, anti-convulsant, anti-cholinergics, and promotility drug within 2 weeks, genetic problems such as galactose intolerance, lactase deficiency, and glucose-galactose malabsorption, any malignancy within 5 years, pregnant or lactating females, and females of childbearing age not using contraception.
Study design
This study was a multicenter, randomized, double-blind, exploratory clinical trial conducted in South Korea from December 2014 to September 2016. Patients meeting the study criteria underwent screening endoscopy, laboratory tests, and urea breath test (UBT) for H. pylori. Eligible patients were randomly assigned to either irsogladine maleate (Gaslon N® OD, 2 mg twice a day) or placebo treatment group. Irsogladine maleate and placebo were taken twice daily for 8 weeks, concurrent with NSAIDs or aspirin.
An independent statistical office (Seoul CRO Co. Ltd., Seoul, Korea) performed permuted stratified block randomization using block size 2 or 4 to stratify study site. They used SAS version 9.2 (SAS institute Inc., Cary, NC, USA) for sequence generation to stratify participating center. Random sequence was sent to each center via an interactive web-based response system to ensure allocation concealment. Patients with a final compliance of less than 80% were excluded from per protocol (PP) analysis. Among 100 eligible patients, 88 subjects were randomly assigned to this study. Of these 88 subjects, 87 (98.86%) were received the clinical trial drug. However, only 76 (86.36%) completed the clinical trial. Reasons for withdrawal included withdrawal of consent (n = 8, 9.09%), and decision of the investigator (n = 3, 3.41%). Of 88 randomized patients (100%), 87 (98.86%) were included in the safety arm, of which 76 (86.36%) were included in the intention-to-treat (ITT) group while 11 patients (12.50%) were excluded due to misuse of medication and misuse of IP. A total of 65 patients (73.86%) were admitted to PP group. Fig. 1 presents a flowchart of patient progression through the study with reasons for premature discontinuation. The subject visited each center for follow-up at week 4 and week 8.
Number of target subjects and basis of calculation
In order to see the expected effect between the test group and the control group, the incidence of peptic ulcer should be predicted based on previous studies. However, since there is no previous study on irsogladine maleate, we used previous studies regarding difference in the incidence of peptic ulcer expected to be statistically tested [23-30]. In this study, we referred to other studies conducted on patients with peptic ulcer due to prolonged administration of NSAIDs. The incidence of peptic ulcers was 21.7% in the placebo group and 1.4% in the group prescribed 200 μg of misoprostol for 12 weeks for prevention and treatment of stomach and duodenal ulcer as an analogue of prostaglandin E1 [24]. Based on this, it was possible to calculate that 44 subjects were required for each group using the following formula by setting the first kind error of 5%, 80% statistical power, the test of both sides, and a dropout rate of 20%.
Upper GI endoscopy and measurement of malondialdehyde level
Endoscopy and malondialdehyde (MDA, a lipid peroxidation product) level measurements were performed at Visit 1 (day 0) and Visit 3 (8 weeks ± 20 days). Standard and near or far range photo takings were performed according to guidelines for upper GI endoscopy. Diagnosis and evaluation of endoscopic photographs were conducted by the investigator of each institution. Separately, endoscopic photographs were collected at the center using a portable storage device. Diagnosis and evaluation were carried out by two independent evaluators.
MDA was measured as a surrogate marker to assess the degree of oxidative stress and antioxidant effect of the test drug. However, MDA level measurement was omitted for subjects whose endoscopic examinations were waived because endoscopic examination of visit 1 (day 0) was confirmed within 4 weeks of the internal examination and enough image results were confirmed to be suitable for participating in this study. MDA level was measured by biopsy in subjects who were confirmed to have ulcer by endoscopy. Cryotube for biopsy was labelled and provided to each institution. In principle, each institution should store the collected sample in a deep freezer for up to 2 months. These samples were then sent to the Central Lab (Seoul National University Bundang Hospital, Seongnam, Korea). Sent samples were used to measure level of MDA, a representative marker of peroxidation products known as the cause or result of GI tissue damage, by homogenization. Since there was no sample remaining after MDA measurement, the sample was automatically discarded. MDA was measured in tissue homogenate using ELISA kit (Sigma-Aldrich, St. Louis, MO, USA) [31]. MDA was extracted by homogenizing isolated gastric mucosa (10 mg) in 300 μL of MDA lysis buffer containing 3 μL of BHT (100×) followed by centrifugation at 13,000 ×g for 10 minutes. Thiobarbituric acid solution was added into each vial containing 200 μL of standards and sample followed by incubation at 95°C for 60 minutes. After cooling to room temperature in an ice bath for 10 minutes, 200 μL was taken from each mixture into a 96-well plate for analysis.
Study assessments
Efficacy
Each subject underwent an upper GI endoscopy to establish a baseline and again after the 8-week treatment period. Primary efficacy evaluation variables were incidence of peptic ulcer and gastric mucosal protection rate as a ratio of modified Lanza score (MLS) of 2 to 4 between test and control groups at 8 weeks (Table 1) [32,33]. Secondary efficacy evaluation variables were the number of acute erosions confirmed by endoscopy at 8 weeks, MDA level change, comparison of edema score, redness score, and hemorrhage score at 8 weeks between the test and control groups (Table 1) [34], and effective rate of subjective symptoms. The level of MDA was measured for two biopsy samples obtained from the greater curvature of mid antrum at initial and 8-week endoscopies. Two more biopsy samples were obtained at the ulcer edge in case of gastric ulcer occurrence for MDA level measurement.
Safety
Safety analysis was based on the safety analysis group and the number of adverse reactions and rescue drug use. In addition, the presence or absence of adverse events was compared using appropriate statistical analysis according to characteristics of variables.
Statistical analysis
Data obtained from subjects were analysed in three forms: safety group, ITT group, and PP group. Safety analysis included all data from randomly assigned subjects who took the study drug in the analysis. In the analysis of the ITT group, all subjects who had data on primary efficacy evaluation parameters after administration of the clinical trial drug were included in the analysis. The incidence of peptic ulcer by gastroscopy was analysed if missing data were obtained at a certain point in time. When the subject was dropped before the clinical trial was terminated, the latest data were obtained at that time (last observation carried forward method). Analysis of the PP group means analysis of the subjects included in the ITT analysis from the data obtained from the subjects completed according to the clinical trial plan. In principle, ITT method was used as the main method for validity data. Additional PP analysis was performed when necessary. Safety data were principally based on safety analysis. Data on efficacy were used to perform subanalyses based on UBT test positive and negative results, and NSAIDs or aspirin naive and existing users.
Primary efficacy parameters were presented as frequency and ratio in each group. Chi-square test or Fisher's exact test was performed at significance level of 5% to test differences between groups. Secondary efficacy variables were descriptive statistics for continuous data. Paired t test or Wilcoxon signed rank test was used to test differences between baseline and follow-up visits for both test and control groups. Independent t test was performed to determine differences between groups. Frequency, proportion, and 95% confidence interval (CI) were presented for discrete data and chi-square test or Fisher exact test was performed to determine differences between groups. The percentage of subjects who developed adverse reactions and 95% CI were presented for each group and chi-square test or Fisher's exact test was performed when difference between the test group and the control group was needed.
Ethics statement
This clinical trial was approved by the Institutional Review Board (IRB) of each participating institution (n = 14) (IRB number of Seoul National University Bundang Hospital: B-1404/245-006).
RESULTS
Baseline characteristics of study participants
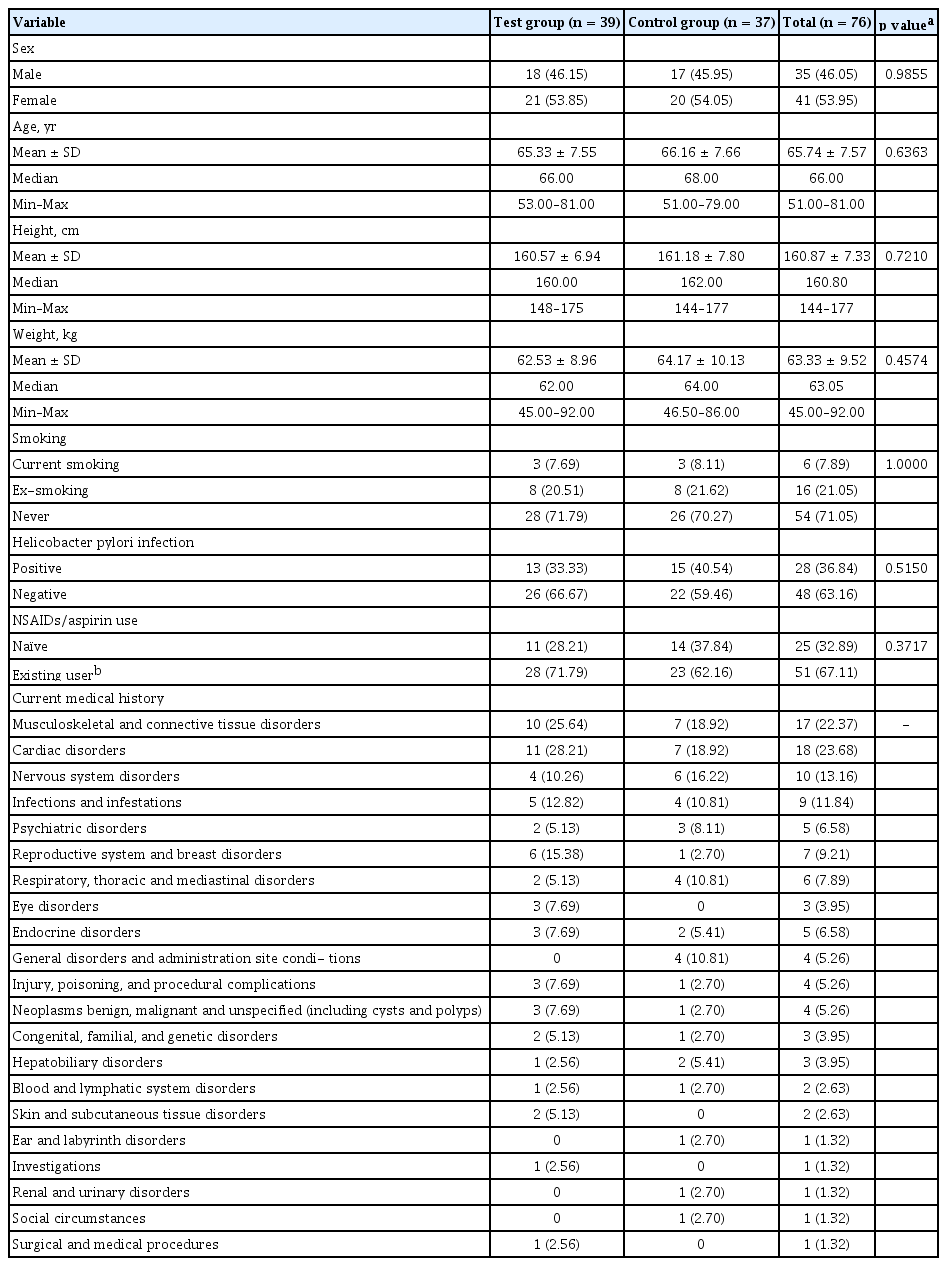
In the ITT group, there were 35 males (46.05%) and 41 females (53.95%). Their mean age, height, and body weight were 65.74 ± 7.57 years, 160.87 ± 7.33 cm, and 63.33 ± 9.52 kg, respectively. There was no statistically significant difference in any demographic characteristics including H. pylori infection status and history of NSAIDs or aspirin use between the two groups (Table 2). Medical history of subject was examined and presented as System Organ Class (SOC) and Preferred Term (PT) according to Medical Dictionary for Regulatory Activities (MedDRA) version 19.0 (MedDRA, McLean, VA, USA). There was no statistically significant difference in medical history between treatment groups, including 15 cases of past history of GI disorders in 13 patients (17.11%) and 28 cases of past medical history in 18 patients (23.68%) in the ITT group (p = 0.1358). Current history of continued use of NSAIDs or aspirin for more than 8 weeks was confirmed in 249 cases of 76 patients of ITT group (100%), including cases of vascular disorders (49 cases in 49 patients, 64.47%), metabolic and nutritional disorders (45 cases in 39 patients, 51.32%), musculoskeletal and connective tissue disorders (22 cases in 17 patients, 22.37%), and cardiac disorders (20 cases in 18 patients, 23.68%), and nervous system disorders (12 cases in 10 patients, 13.16%). Current status of GI disorders was found in 33 cases of 26 patients (34.21%). Detailed medical history for the use of NSAIDs or aspirin was shown in Table 2.
Efficacy
Primary and secondary efficacy parameters in ITT group and PP group were analysed. ITT group was divided into NSAIDs or aspirin naive and NSAIDs or aspirin participating group with more than 7 days of wash-out period, H. pylori-positive group, and H. pylori-negative group.
Primary efficacy assessment
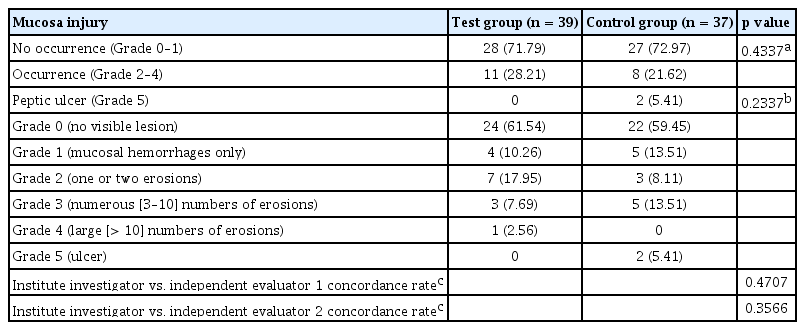
The ITT population had 76 patients (39 in the test group and 37 in the placebo group). Primary efficacy endpoint was MLS score of 5 and MLS score of 2 to 4 at 8 weeks. Results of endoscopic finding by institutional investigators were confirmed by two independent evaluators and consistency in MLS standard score was confirmed. Cohen’s weighted κ value for the agreement between institute investigator and independent evaluator 1 was 0.4707 (moderate concordance) and that between institute investigator and independent evaluator 2 was 0.3566 (fair concordance).
(1) Incidence of peptic ulcer: At the end of the 8th week after administering the test drug, the percentage of patients with peptic ulcer (MLS 5) was assessed. As a result, no subject (0%) in the test group and two subjects (5.41%) in the control group were found to have gastric ulcer (one case of peptic ulcer in aspirin-naive patient and one case in patient who was taking aspirin after abstinence for more than 7 days). Both cases occurred in H. pylori-negative control patients. There was no statistical significance between groups (Table 3). Subgroup analysis did not show any statistical significance between groups either.
(2) Incidence of MLS: In endoscopic examination at 8 weeks after administering the test drug, 11 subjects (28.21%) in the test group and eight patients (21.62%) in the control group were found to have peptic inflammation with MLS of 2 to 4. There was no statistically significant difference in incidence of peptic inflammation with MLS of 2 to 4 between the two groups (Table 3). Subgroup analysis did not show any statistically significant difference in incidence of peptic inflammation with MLS of 2 to 4 between groups either.
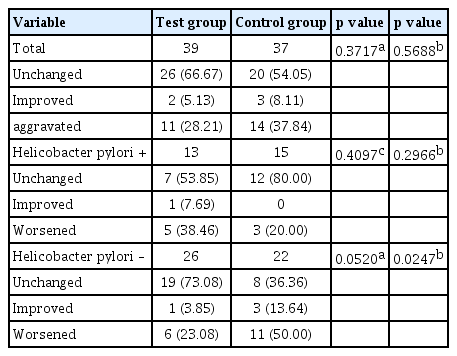
Incidence or aggravation of peptic ulcer/inflammation by NSAIDs or aspirin treatment at 8 weeks after administering the test drug was assessed by MLS grade change between before and after test drug administration. As a result, changes in the test group were improvement in two (5.13%), unchanged in 26 (66.67%), and worsened in 11 (28.21%) patients. Changes in the control group were improvement in three (8.11%), unchanged in 20 (54.05%), and deteriorated in 14 patients (37.84%) (Table 4). Therefore, peptic ulcer/inflammation deterioration was prevented slightly by administration of the test drug. However, the difference between the two groups was not statistically significant (unchanged + improvement vs. worsened, p = 0.3717; unchanged vs. improved vs. worsened, p = 0.5688) (Table 4). In subgroup analysis, for all subgroups except H. pylori-positive group, administration of test drug showed a tendency to prevent peptic ulcer/inflammation worsening. H. pylori-negative subgroup analysis showed that changes in the test group were improvement in one (3.85%), unchanged in 19 (73.08%), and deteriorated in six patients (23.08%). Those in the control group were improvement in three (13.64%), unchanged in eight (36.36%), and worsened in 11 patients (50.00%). Differences between groups were statistically significant (unchanged + improvement vs. worsened, p = 0.0520; unchanged vs. improvement vs. worsened, p = 0.0247) (Table 4).
Secondary efficacy assessment
(1) Number of acute erosions: The number of acute erosions confirmed by endoscopy at 8 weeks as a secondary efficacy variable was 0.79 ± 1.82 in the stomach of the test group, 0 in the duodenum of the test group, 1.05 ± 1.96 in the stomach of the control group, and 0.14 ± 0.82 in the duodenum of the control group. Therefore, a total of 0.79 ± 1.82 and 1.19 ± 2.42 acute erosions were identified in the test group and control group, respectively. The number of acute erosions tended to be smaller in the test drug group compared to that in the control group. However, the difference between the two was not statistically significant (p = 0.5884) (Supplementary Table 1). In subgroup analysis, for all subgroups except H. pylori-positive group, test group showed a tendency to have a smaller number of acute erosions compared to the control group. However, the difference between the two groups was not statistically significant. The number of acute erosions in the stomach and duodenum determined by institutional investigator showed fair (0.610 or 0.564) and good (0.663 or 0.692) agreement with the number of acute erosion evaluated by independent evaluator 1 or 2, respectively (Supplementary Table 1).
(2) MDA level changes based on endoscopic biopsy: Changes in MDA levels measured at 8 weeks versus baseline were –0.22 ± 0.94 nmol/μL in the test group and –0.05 ± 0.70 nmol/μL in the control group. However, the reduction in MDA level was not statistically significant in either group. The reduction in MDA level in the test group at 8 weeks after administration of the test drug was about four times higher than that in the control group. However, the difference between the two was not statistically significant (p = 0.4098) (Supplementary Table 2). Similar trends were observed in all subgroups.
(3) Efficacy against edema: After the treatment, changes for edema in the test group were improvement in one (2.56%), unchanged in 36 (92.31%), and worsening in two patients (5.13%). Changes for edema in the control group were improvement in one (2.70%), unchanged in 31 (83.78%), and deteriorated in five patients (13.51%). However, difference was not statistically significant (unchanged + improved vs. worsened, p = 0.2562; unchanged vs. improved vs. worsened, p = 0.6229) (Table 5). Subgroup analysis showed that administration of test drug tended to prevent edema in all subgroups. In particular, H. pylori-negative subgroup analysis showed that 26 patients (100%) in the test group were unchanged. However, in the control group, one patient (4.55%) was improved, 17 (77.27%) were unchanged, and four (18.18%) were worsened (unchanged vs. improved vs. worsened, p = 0.0154; unchanged + improved vs. worsened, p = 0.0376). Their differences were statistically significant (Table 5). Results of edema evaluation by institutional investigators showed fair or poor agreement with results determined by two independent evaluators.
(4) Efficacy against redness: The protection efficacy of test drug against NSAIDs or aspirin-induced redness was evaluated by determining the degree of flares (normal, mild, moderate, severe) through gastroscopy at baseline and the 8th week. As a result, changes in NSAIDs or aspirin-induced redness in the test group were improved in eight (20.51%), unchanged in 21 (53.85%), and worsened in 10 patients (25.64%). In the control group, those changes were improved in eight (21.62%), unchanged in 19 (51.35%), and worsened in 10 patients (27.03%). There was no statistically significant difference in redness changes between the two groups (unchanged + improvement vs. worsening, p = 0.8909; unchanged vs. improved vs. worsening, p = 0.9766). There was no statistically significant difference in subgroup analysis between the two groups either. Results of redness evaluated by institutional investigators showed fair or poor agreement with results determined by two independent evaluators.
(5) Efficacy against haemorrhage: Baseline and 8th week gastroscopies were performed to evaluate the degree of hemorrhage (none, 1 lesion, 2 to 5 lesions, 6 to 10 lesions, or more than 10 lesions). Prevention of NSAIDs or aspirin-induced hemorrhage was evaluated. As a result, changes in hemorrhage in the test group were improved in 0 (0%), unchanged in 34 (87.18%), and aggravated in five patients (12.82%). In the control group, these changes were improved in two (5.41%), unchanged in 31 (83.78%), and aggravated in four patients (10.81%). There was no statistically significant difference in changes of hemorrhage between the two groups (unchanged + improved vs. aggravated, p = 1.0000; unchanged vs. improved vs. worsened, p = 0.4361). In subgroup analysis, there was statistically significant difference in hemorrhage changes between the two groups in the H. pylori-positive group (unchanged + improved vs. worsened, p = 0.0349; unchanged vs. improved vs. worsened, p = 0.0349). However, the number of subjects used in this study was small. In other subgroups, there was no statistical significance in hemorrhage changes between the two treatment groups. Results of hemorrhage evaluation by institutional investigators showed fair or poor agreement with results obtained by two independent evaluators.
(6) Efficacy against subjective symptoms: The frequency and severity of GI symptoms were evaluated using Korean version of the Nepean Dyspeptic Index (NDI). NDI awareness symptom evaluation scale consisted of 10 GI symptoms. The frequency of each question was rated 0 to 4 points and the severity was scored 0 to 3 points. The higher the score, the greater the frequency and symptoms. Total score changes of subjective symptom frequency between the 8th week and baseline in test group were improved in 10 (25.64%), unchanged in 13 (33.33%), and deteriorated in 16 patients (41.03%). In the control group, these changes were improved in 12 (32.43%), unchanged in 15 (40.54%), and aggravated in 10 patients (27.03%). There was a trend of increased subjective symptom frequency in the test group. However, the difference between the two groups was not statistically significant (unchanged + improved vs. worsening, p = 0.1985; unchanged vs. improved vs. worsening, p = 0.4365). All subgroups showed similar trends.
As a result of evaluating total score changes for the degree of subjective symptom at the 8th week compared to those at baseline based on case rate, changes in subjective symptoms were improved in eight (20.51%), unchanged in 16 (41.03%), and deteriorated in 15 patients (38.46%) in the test group. In the control group, these changes were improved in 11 (29.73%), unchanged in 15 (40.54%), and aggravated in 11 patients (29.73%). There was no statistically significant different between the two treatment groups (unchanged + improved vs. worsening, p = 0.4226; unchanged vs. improved vs. worsening, p = 0.5858).
Safety
A total of 19 adverse events occurred in 16 out of 87 patients in the safety group. There was no statistically significant difference in adverse events between the two treatment groups. Two serious adverse events (10.53%) occurred. Patients were hospitalized due to chest discomfort and influenza symptoms. However, there was no causal relationship between the two events and the clinical trial drug. GI disorders were the most common (six cases, 6.90%). At 4 and 8 weeks after administration of the drug for clinical trial, rescue drug was administered 6.55 ± 11.23 times in the test group and 6.92 ± 7.98 times in the control group at 4 weeks and was administered 5.90 ± 8.16 times in the test group and 4.70 ± 6.94 times in the control group at 8 weeks. There was no significant difference in the number of rescue drug administration between the two groups.
DISCUSSION
The present study evaluated the efficacy and safety of irsogladine maleate for preventing NSAIDs or aspirin-induced peptic ulcer and gastritis in patients who were more than 50 years old. There were two cases of peptic ulcer in the placebo group and no peptic ulcer in the test group. However, the difference in the incidence of peptic ulcer between the two groups did not reach statistical significance mainly due to small number of subjects used in this study. Another significant finding was that changes in MLS score (p = 0.0247) and edema score (p = 0.0154) were statistically significant between before and after treatment in H. pylori-negative patients. This means that the protective effect of irsogladine maleate is prominent when subjects are H. pylori-negative. H. pylori-infected gastric mucosa or gastric epithelial cell lines have shown inhibition in HKα promotor activity of endogenous or transfected H,K-ATPase [35]. Chronic H. pylori infection in the antrum can up-regulate gastrin and subsequently elevate acid secretion while chronic infection in the corpus can lead to impaired acid secretion by direct suppression of H,K-ATPase or involvement of cytokines such as interleukin-1β or tumor necrosis factor-α [36]. Therefore, direct inhibition of H,K-ATPase, indirect inhibition through cytokines, and loss of parietal cells by ongoing inflammation are three mechanisms associated with low acid secretion in chronic corpus predominant gastritis [37]. In addition, only 40% of H. pylori-negative peptic ulcer Korean patients who received continuous PPI infusion reached target pH > 6 for more than 60% of the time, which was significantly lower than that in the H. pylori-positive group at 87.5% (p = 0.026) [38]. Taken together, these results suggest that adequate acid inhibition might be difficult in H. pylori-negative patients. However, significant protective effect of irsogladine maleate in H. pylori-negative subjects was found in the present study. This suggests that, when NSAIDs or aspirin is unavoidable in patients who are over 50 years old, gastroprotective drug should be added, especially in the absence of H. pylori-induced buffer effect. Another supportive evidence for this assumption was that two subjects who developed gastric ulcers while taking NSAIDs or aspirin in the placebo group were H. pylori-negative subjects. Our results favour results of previous reports showing that H. pylori gastritis is not a risk factor for gastroduodenal damage in subjects taking NSAIDs or aspirin [39-43].
The mechanism of action of irsogladine maleate (Gaslon N® OD) is known to cause mucoprotection through activating intercellular communication and increasing gastric mucosal blood flow, anti-inflammatory action, and maintenance of mucosal hydrophobicity [44-46]. However, primary or secondary outcomes were not significantly different between test group and control groups, although we recruited patients at age of more than 50 years old with an average age of 65.74 years. This might be due to the fact that insufficient number of subjects were recruited to find difference between the two groups. The incidence of peptic ulcer (21.7%) in the hypothetical placebo group was somewhat high. However, it did not sufficiently reflect the recent tendency of decreasing prevalence of peptic ulcer [47]. Moreover, the protective effect of misoprostol for peptic ulceration was also relatively high in the reference study [24]. In spite of this critical limitation, two cases in the placebo group showed peptic ulcer while none in the test group showed peptic ulcer. These two cases of peptic ulcer were both H. pylori-negative patients. In addition, H. pylori-negative group showed significant changes in MLS score (p = 0.0247) and edema score (p = 0.0154) between before and after the treatment. These results suggest that irsogladine maleate might have potential as a protective agent against NSAIDs or aspirin-induced peptic ulcer and gastritis.
KEY MESSAGE
1. The widespread use of nonsteroidal anti-inflammatory drugs (NSAIDs) raises the risk of drug side effects, including gastrointestinal damage. The risk of gastric mucosal defects (erosion or petechiae) in patients with long-term use of NSAIDs has been reported to be about 50%.
2. Misoprostol and proton pump inhibitor preparations are usually recommended to prevent NSAID or aspirin-induced peptic ulcer and gastritis, especially for the elderly. However, there are limitations of their usage such as abdominal pain and longterm use related various side effects, respectively.
3. Irsogladine maleate (Gaslon N® OD, Taejoon Pharm Co. Ltd.) is an enhancer of gastric mucosal protective factors. Our study suggests that its potential as a protective agent against NSAIDs or aspirin-induced peptic ulcer and gastritis in Helicobacter pylori-negative subjects.
Notes
This study was funded by a grant from Taejoon Pharm Co. Ltd., Seoul, South Korea.