Clinical factors affecting progression-free survival with crizotinib in ALK-positive non-small cell lung cancer
Article information
Abstract
Background/Aims
Although crizotinib is standard chemotherapy for advanced anaplastic lymphoma kinase (ALK)-positive non-small cell lung cancer (NSCLC), clinical factors affecting progression-free survival (PFS) have not been reported. The purpose of this study was to identify clinical factors affecting PFS of crizotinib and develop a prognostic model for advanced ALK-positive NSCLC.
Methods
Clinicopathologic features of patients enrolled in PROFILE 1001, 1005, 1007, and 1014 (training cohort) were reviewed. We conducted multivariate Cox analysis for PFS and overall survival (OS) in the training cohort (n = 159) and generated a proportional hazards model based on significant clinicopathologic factors, and then validated the model in an independent validation cohort (n = 40).
Results
In the training cohort, the objective response rate was 81.5%. Median PFS and OS from the start of crizotinib were 12.4 and 31.3 months, respectively. Multivariate Cox analysis showed poor performance status, number of metastatic organs (≥ 3), and no response to crizotinib independently associated shorter PFS. Based on a score derived from these three factors, median PFS and OS of patients with one or two factors were significantly shorter compared to those without these factors (median PFS, 22.4 months vs. 10.5 months vs. 6.5 months; median OS, not reached vs. 29.1 months vs. 11.8 months, respectively; p < 0.001 for each group). This model also had validated in an independent validation cohort.
Conclusions
Performance status, number of metastatic organs, and response to crizotinib affected PFS of crizotinib in ALK-positive NSCLC. Based on these factors, we developed a simple and useful prediction model for PFS.
INTRODUCTION
Anaplastic lymphoma kinase (ALK) is well validated tyrosine kinase target in non-small cell lung cancer (NSCLC) [1]. Echinoderm microtubule associated protein like 4 (EML4)-ALK fusion was first identified in NSCLC [2] and is found in approximately 3% to 5% of cases of NSCLC, defining a distinct molecular subtype of NSCLC [3-5]. Crizotinib is a multi-targeted small-molecule ALK inhibitor approved for the treatment of advanced ALK-positive NSCLC [1]. Randomized phase III trials have clearly shown that crizotinib significantly improves progression-free survival (PFS) when compared with conventional cytotoxic chemotherapy for previously treated or treatment-naïve advanced ALK-positive NSCLC (PROFILE 1007 and PROFILE 1014) [6,7]. Crizotinib is currently the standard chemotherapy for ALK-positive NSCLC.
Even though the response rate of ALK-positive NSCLC to crizotinib is much higher than conventional cytotoxic chemotherapy, all patients who initially respond to crizotinib eventually develop acquired resistance and disease progression. Because of acquired resistance, clinicians have difficulty in predicting when tumor progression will occur during crizotinib therapy in individual patients. In this regard, it is clinically important to be able to predict the timing of disease progression during crizotinib treatment.
However, to date, clinicopathologic factors that significantly affect PFS of crizotinib have not been reported. Besides the delicate molecular work to understand primary and secondary resistance to crizotinib [8,9], pragmatic models to predict tumor progression with crizotinib might help the clinician to better determine the prognosis of patients before or during crizotinib treatment. The objectives of this study were to: (1) identify clinical factors affecting PFS of crizotinib and (2) develop a practical model for predicting disease progression in ALK-positive NSCLC with crizotinib.
METHODS
Study population
Medical records of patients diagnosed with ALK-positive NSCLC and receiving crizotinib were analyzed. We developed two independent cohorts (Supplementary Fig. 1). A training cohort consisted of patients who were enrolled in PROFILE 1001 (NCT00585195, n = 50) [1], PROFILE 1005 (NCT00932451, n = 78) [10], PROFILE 1007 (NCT00932893, n = 12) [6], and PROFILE 1014 (NCT01154140, n = 31) [7] clinical trials. An independent validation cohort included patients given compassionate use crizotinib (n = 3) or treated with crizotinib after Korean Food and Drug Administration approval (n = 37). ALK positivity in the training cohort was defined by ALK break-apart fluorescence in situ hybridization (FISH) assay, as described for each clinical trial [1,6,7,11]. Experienced pathologists (Y.K.J. and D.H.C.) from the Seoul National University Hospital confirmed NSCLC as ALK-positive when 15% or more of tumour cells showed split and/or isolated 3′ signals in 50 analyzed cells [12] using a Vysis ALK Break Apart FISH Probe Kit (Abbott Molecular Inc., Des Plaines, IL, USA) for patients in the validation cohort. Age, sex, smoking history, initial performance status by Eastern Cooperative Oncology Group (ECOG), metastatic organs (such as brain, lung-to-lung, bone, liver, lymph node, and pleura [13]) before crizotinib treatment, and crizotinib response were analyzed.
Treatment and response evaluation of crizotinib
After baseline imaging with computed tomography of the chest and abdomen and magnetic resonance imaging of the brain (if previously known brain metastases or suspected by neurologic symptoms), patients received oral crizotinib at a dose of 250 mg twice daily. Follow-up tumour assessment was performed every 6 to 12 weeks during treatment until progression. Tumour assessment was based on Response Evaluation Criteria In Solid Tumours (RECIST) 1.0 [14] for patients enrolled in PROFILE 1001 and by RECIST 1.1 [15] for others. PFS was calculated from the start date of crizotinib treatment to the date of disease progression by RECIST criteria, as confirmed by imaging, death, or the last follow-up date if censored. Overall survival (OS) was measured from the initiation of crizotinib treatment until death or the last follow-up date, if censored.
Statistical analysis
Univariate and multivariate Cox proportional hazard regression analyses were performed, summarizing the hazard ratio (HR) and 95% confidence interval (CI) for each group. Survival analyses were carried out according to the Kaplan-Meier method with the log-rank test to assess differences between the groups. All reported p values are two-sided and considered significant if p < 0.05. All statistical analyses were carried out using STATA version 12 (StataCorp LP, College Station, TX, USA).
Ethics
The study protocol was approved by the Institutional Review Board of Seoul National University Hospital (approval number: H-1411-098-628) and was conducted in accordance with the Principles of the Declaration of Helsinki. Informed consent was waived by the IRB, since it was retrospective analysis and did not affect the clinical outcome of the subject.
RESULTS
Patient characteristics by treatment type and response
Baseline clinicopathologic characteristics of the training and validation cohorts are summarised in Table 1. In the training cohort, the median age of patients given crizotinib was 54 years, and 91 (57.2%) were female. The majority of patients (79.9%) were initially diagnosed with stage IIIB/IV metastatic lung cancer, and the mean number of metastatic organs prior to crizotinib treatment was 2.77 (range, 1 to 6). Twenty-six patients (16.4%) were given crizotinib as first-line treatment. Among the 157 evaluable patients, the objective response rate (complete and partial responses) was 81.5% (95% CI, 75.4% to 87.6%). During a median follow-up of 45.5 months (range, 3.9 to 74.5), 136 patients (85.5%) experienced progression with crizotinib; 81 (50.9%) had died at the time of final analysis (January 11, 2015). Median PFS of crizotinib was 12.4 months (95% CI, 10.2 to 15.6), and OS from the start of crizotinib was 31.3 months (95% CI, 26.2 to 42.3).
Patient characteristics were not different between the training and validation cohorts, except more patients in the validation cohort had poorer performance status (p = 0.022) and were more likely to receive crizotinib as their first-line treatment (p = 0.021). Median follow-up duration in the validation cohort was 14.7 months, which was significantly shorter than the training cohort (p < 0.001). Kaplan-Meier analyses of PFS and OS for training and validation cohorts are shown in Fig. 1.

Progression-free survival and overall survival after crizotinib treatment. Kaplan-Meier curves for progression-free survival (A) and overall survival (B) according to training cohort (n = 159) and validation cohort (n = 40) are shown. mPFS, median progression-free survival; CI, confidence interval; HR, hazard ratio; mOS, median overall survival; NR, not reached.
We examined the hazard function for progression. Supplementary Fig. 2 shows kernel estimates of the hazard functions for progression in the training cohort. Crizotinib-treated ALK-positive NSCLC patients had a pattern of consistent progression without a peak risk of progression during 24 months. This finding suggested that disease progression can develop evenly during crizotinib.
Univariate and multivariate Cox proportional hazard regression analyses of PFS and OS
Univariate and multivariate Cox regression analyses of PFS and OS in the training cohort were performed (Table 2). Univariate Cox regression analysis showed poor performance status (ECOG status 2 or 3), ≥ 3 metastatic organs, baseline brain metastasis, and no response to crizotinib (stable or progressive disease) significantly affected shorter PFS and OS. In multivariate Cox regression analysis, three factors except baseline brain metastasis were independently correlated with PFS and OS.
A predicting model to predict PFS and OS
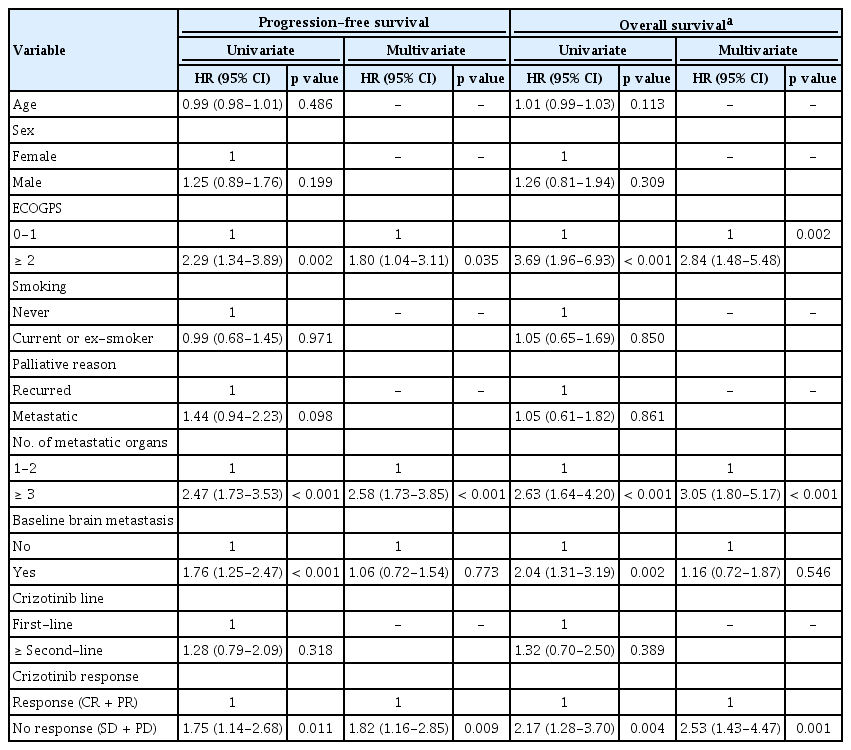
In multivariate Cox regression analysis, three independent prognostic factors (ECOG performance status, number of metastatic organs, and crizotinib response) showed similar HR (range, 1.81 to 2.64). Hence, we developed a simple and practical model to predict survival by allocating 1 point for each factor. For example, a patient with an ECOG status (= 3), stable disease, and four metastatic organs had 3 points. We excluded the patients who could not evaluate crizotinib response due to early follow-up loss in this model. Based on points derived from significant clinical factors of ECOG status 2 to 3, number of metastatic organs ≥ 3, and no response to crizotinib, patients were divided into three groups: those with 0 point, 1 point, and 2 to 3 points (n = 59, n = 73, and n = 25, respectively). Median PFS of patients with 0 point was 22.4 months (95% CI, 16.6 to 29), which was significantly prolonged compared to those with 1 point (median PFS 10.5 months; 95% CI, 9 to 12.6; p < 0.001), and 2 to 3 points (median PFS 6.5 months; 95% CI, 4 to 10.2; p < 0.001) (Fig. 2A). Moreover, median OS of patients with 0 point was not reached and was also significantly prolonged compared with those patients with 1 point (median OS 29.1 months; 95% CI, 22.7 to 38.9; p < 0.001), and 2 to 3 points (median OS 11.8 months; 95% CI, 4.6 to 20.6; p < 0.001) (Fig. 2B).
Survival analysis according to a proportional hazards model in training cohort. Kaplan-Meier curves for progression-free survival (A) and overall survival (B) after crizotinib treatment in the training cohort according to a proportional hazards model are shown. Score indicates how many factors were present (i.e., Eastern Cooperative Oncology Group performance status, same or more than 2; number of metastatic organs, same or more than 3 organs; and no response by crizotinib treatment). mPFS, median progression-free survival; CI, confidence interval; HR, hazard ratio; mOS, median overall survival.
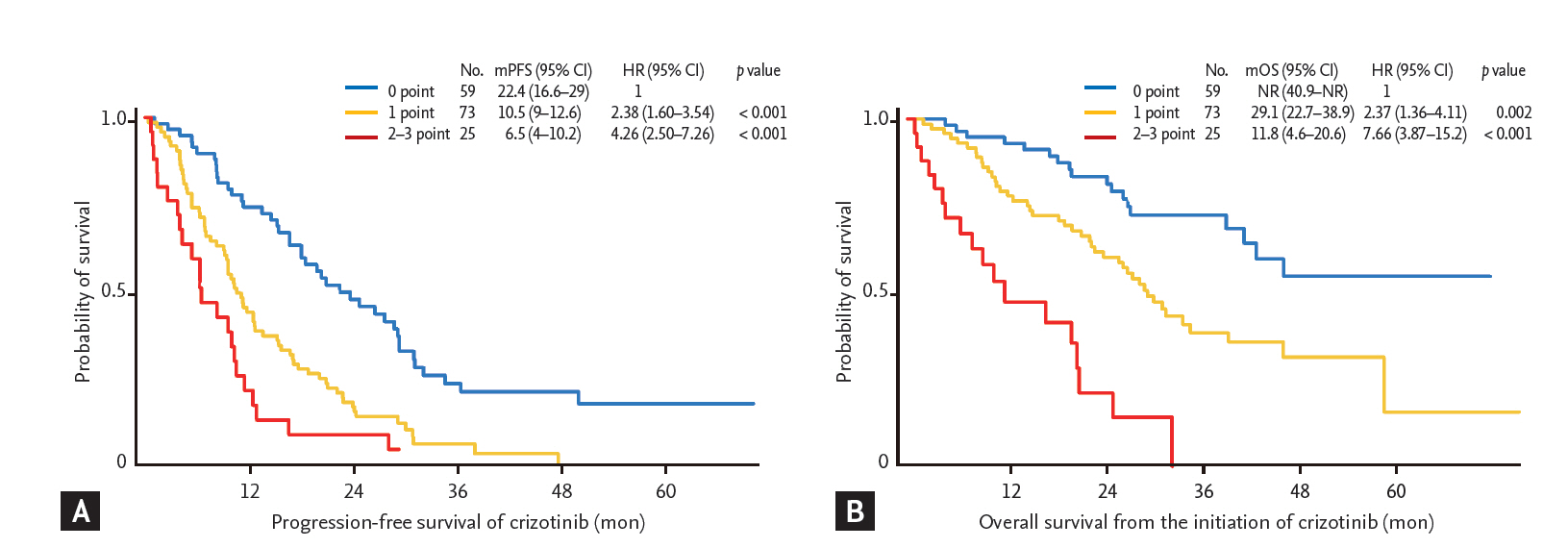
This model also fitted and retained statistical significance in an independent validation cohort, even though number of patients included in the validation cohort was relatively small (n = 40). Based on the same criteria derived from the Cox regression model analyzed in the training cohort, patients in the validation cohort were also divided into three groups: those with 0 point, 1 point, and 2 to 3 points (n = 15, n = 19, and n = 3, respectively). Median PFS of patients in the validation cohort with 0 point was 25.9 months (95% CI, 16.5 to not reached), which was significantly prolonged compared with those patients with 1 point (median PFS 7.1 months; 95% CI, 4.3 to 15.6; p < 0.001), and 2 to 3 points (median PFS 0.3 months; 95% CI, 0.3 to not reached; p < 0.001) (Fig. 3A). Moreover, median OS of patients in the validation cohort with 0 point was not reached, which was also significantly longer than those patients with 1 point (median OS 17.3 months; 95% CI, 7.9 months to not reached; p = 0.043) and 2 to 3 points (median OS not reached; 95% CI, 0.9 months to not reached; p = 0.019) (Fig. 3B).
Survival analysis according to a proportional hazards model in validation cohort. Kaplan-Meier curves for progression-free survival (A) and overall survival (B) after crizotinib treatment in the validation cohort according to a proportional hazards model are shown. Score indicates how many factors are present (i.e., Eastern Cooperative Oncology Group performance status same or more than 2; number of metastases, same or more than 3 organs; and no response by crizotinib treatment). mPFS, median progression-free survival; CI, confidence interval; HR, hazard ratio; mOS, median overall survival; NR, not reached.
DISCUSSION
In our current study, we found that three clinicopathologic factors (poor performance status [ECOG 2 to 3], ≥ 3 metastatic organs, and no response to crizotinib) were significantly associated with shorter PFS and OS. As disease progression can happen at any time during crizotinib treatment, clinicians have difficulty in predicting when disease progression will occur in individual patients. Hence, predicting disease progression after crizotinib treatment is a clinically important issue. In this study, a simple and practical model to predict PFS of crizotinib was developed and validated in an independent cohort.
Clinicopathologic factors significantly associated with PFS and OS were similar to previously published results and showed that ECOG performance status and tumour burden were correlated with survival of epidermal growth factor receptor tyrosine kinase inhibitor (EGFR TKI) [13,16-20]. Although ECOG performance status is a common prognostic factor for cancer, to the best of our knowledge, its use in crizotinib-treated ALK-positive NSCLC has not been reported. As definite oncogenic addiction of ALK-positive NSCLC was shown in preclinical and clinical studies, one might expect that crizotinib treatment would be effective regardless of ECOG performance status. Moreover, the so called “Lazarus response” after EGFR TKI has been observed in treatment-naïve, poor performance status NSCLC patients with EGFR mutations [21]. Surely, molecular targeted agents can be used in poor performance status patients without significant toxicity and with an expected high response rate. However, PFS of poor performance status patients was shorter than that of good performance status patients regardless of a high response rate. In our current study, performance status significantly affected PFS of crizotinib in ALK-positive NSCLC.
A large number of metastatic organs was also an independent factor associated with an unfavorable prognosis in ALK-positive NSCLC, which is in agreement with a previous study regarding EGFR TKI [16]. Although there is no standard method to reflect overall tumor burden, a large number of metastatic organs can suggest a large tumor burden in individual patients. Cancer cells undergo selective pressure from chemotherapy and undergo a genotypical clonal evolution. In an animal model, a large tumor burden would be more likely to result in genetically complex tumors that would exhibit either primary or secondary resistance to erlotinib [22]. As resistant mutant clones might increase in proportion to tumor burden, the probability of developing resistant clones would be higher in a patient with a large tumor burden, and it is likely that these patients show shorter PFS of crizotinib. A prediction model consisting of ECOG performance status, number of metastatic organs, and response to crizotinib might guide physicians in estimating the prognosis of the individual patient as well as the timing of progression with crizotinib early during treatment. Interestingly, the proportional hazards model developed using multivariate Cox regression analysis of PFS also fitted OS in our current study. Extended survival in a good prognosis group might allow for use of other ALK inhibitors currently under development.
Median PFS in PROFILE 1014, which used crizotinib as a first-line treatment, was 10.9 months in the crizotinib arm, but for PROFILE 1007, which included previously treated patients, was 7.7 months [6,7]. Results of PFS from previous trials would imply that crizotinib treatment as a first-line treatment would be the same or better than second-line treatments. In the current analysis, PFS of first-line crizotinib was not statistically longer than that of ≥ second line crizotinib (HR, 1.28; 95% CI, 0.79 to 2.08; p = 0.321). To show OS benefits by first-line treatment compared to second or more than second-line treatment is not easy. However, the improvement in the quality of life in patients given crizotinib in PROFILE 1014 [7], there is no reason to postpone crizotinib treatment to second or more than second line treatment. As ECOG performance and number of metastatic organ are important, it seems to be better not to postpone crizotinib treatment. Although baseline brain metastasis was significantly associated with poor PFS and OS in univariate Cox analysis, but it was not much as significant in multivariate analysis. Since number of metastatic organ would include baseline brain metastasis, assessing overall tumor burden would be more important compared to considering brain metastasis only when we predict PFS or OS of crizotinib.
Our study has some limitations. First, the true definition of disease progression can be vague, because of some patients have received crizotinib even after evidence of progression according to RECIST criteria [14,15]. Disease progression after TKI does not always mean a development of acquired resistance or true treatment failure [23]. There are several evidences supporting that continuing TKI beyond progression would benefit in EGFR-mutant NSCLC [24,25] and in ALK-positive NSCLC [26,27]. However, it has not been clearly shown in well-designed prospective studies regarding crizotinib treatment beyond progression. To minimize this bias, we defined progression and calculated PFS according to RECIST criteria which have been most commonly used. Second, OS differences using a proportional hazards model could be affected by confounding factors, such as exposure to other second generation ALK inhibitors. Third, although our model was validated using an independent data set, the number of patients included in the validation cohort was relatively small. Therefore, it would be necessary to conduct an external validation of our model using another independent patient population. Despite these limitations, the current study is the first to develop a practical model to predict PFS of crizotinib in ALK-positive NSCLC. In conclusion, we found that three independent clinical factors—ECOG performance status, number of metastatic organs, and response to crizotinib affect PFS of crizotinib in advanced ALK-positive NSCLC. Based on these factors, we also developed simple and useful prediction model for PFS, which could help define patient groups based on prognosis. Further studies are needed for external validation of this prediction model.
KEY MESSAGE
1. To identify clinical factors affecting progression-free survival (PFS) of crizotinib in ALK-positive non-small cell lung cancer.
2. PROFILE 1001, 1005, 1007, and 1014 and independent validation cohort were analysed.
3. Performance status, number of metastasis, and tumor response affected PFS.
Notes
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This study was supported by a grant of the Korea Health Technology R&D Project "Strategic Center of Cell and Bio Therapy for Heart, Diabetes & Cancer" through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare (MHW), Republic of Korea (grant number: HI17C2085).